Session Information
Date: Tuesday, November 12, 2019
Title: 5T090: Osteoarthritis – Clinical II: Novel Therapies (2756–2761)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: There is considerable unmet medical need for an osteoarthritic knee pain (OAKP) treatment that does not require regular daily use, does not carry gastrointestinal risk (e.g., NSAIDs), liver risk (e.g., acetaminophen) or addiction risk (e.g., opiates), and can safely provide long-lasting pain relief after a brief course of treatment. The Sponsor has clinically shown that topical OTC-strength 0.25% (w/w) capsaicin is well tolerated and provides OAKP relief. The Sponsor has now produced in the same vehicle (CGS-200) high potency, 5% (w/w) capsaicin (CGS-200-5) and 1% (w/w) capsaicin (CGS-200-1) topical products to evaluate as treatments for the relief of OAKP using the WOMAC subscales for pain, stiffness and function as well as total WOMAC score.
Methods: This was a multi-center, randomized (1:1:1), double-blind, parallel group, vehicle-controlled trial comparing topical CGS-200-1 or CGS-200-5 versus CGS-200-0 in 122 randomized subjects who had OA of at least one knee according to 1986 ACR criteria and a WOMAC pain score of ≥ 250. Subjects continued ongoing non-prohibited analgesic medication(s) (e.g., oral NSAIDs) at study entry with the expectation that the daily dose was to be maintained throughout the study if possible. Treatment was applied for 1 hour to both knees on 4 consecutive days (Study Days 1-4) by the subject under clinic supervision and then washed off. Clinical efficacy and safety assessments were made on Study Days 5, 19, 35 (the primary endpoint was Day 35 change in WOMAC pain score from baseline), 64, and 94. The modified intent to treat (mITT) subset of subjects was used for all efficacy analyses.
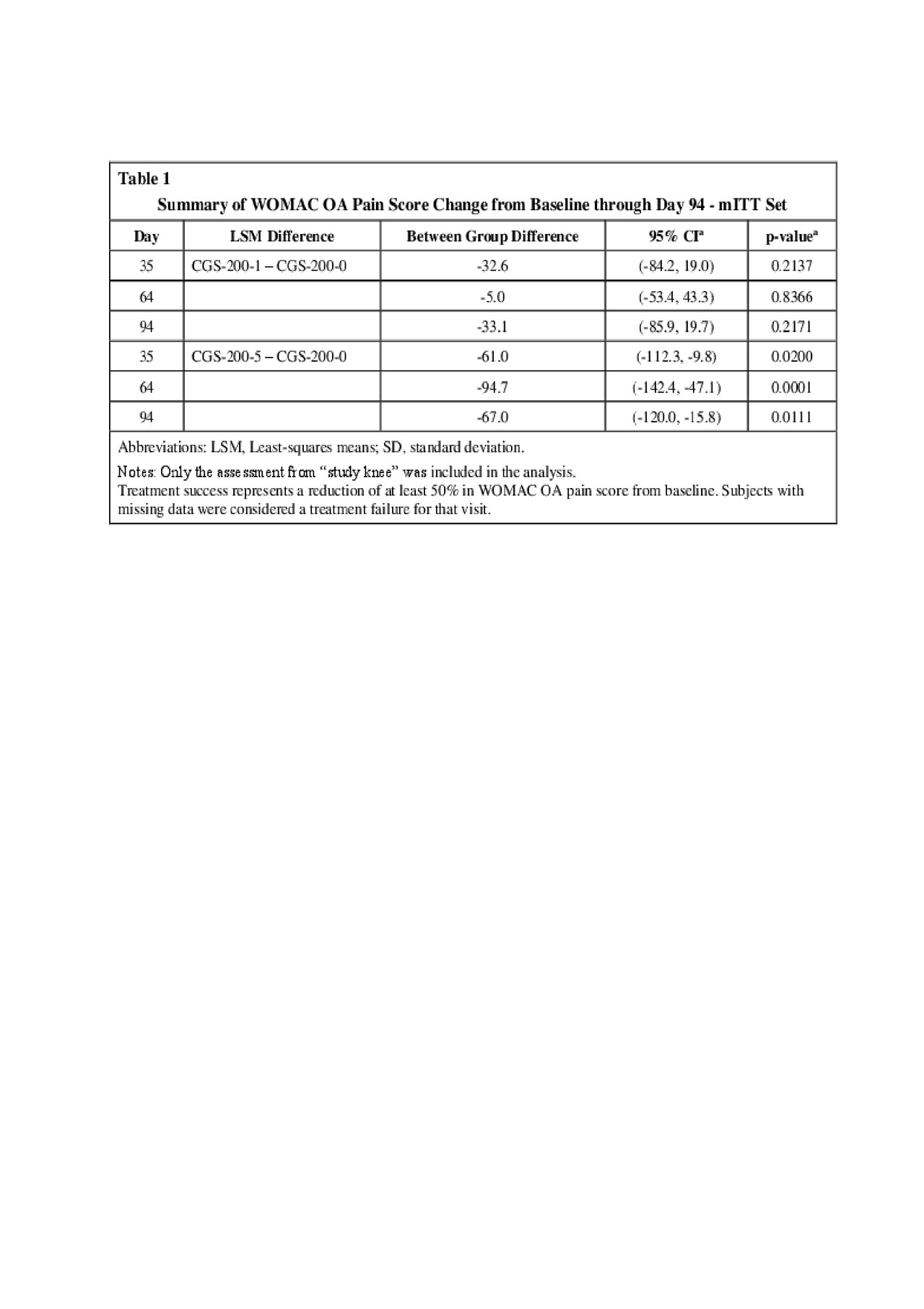
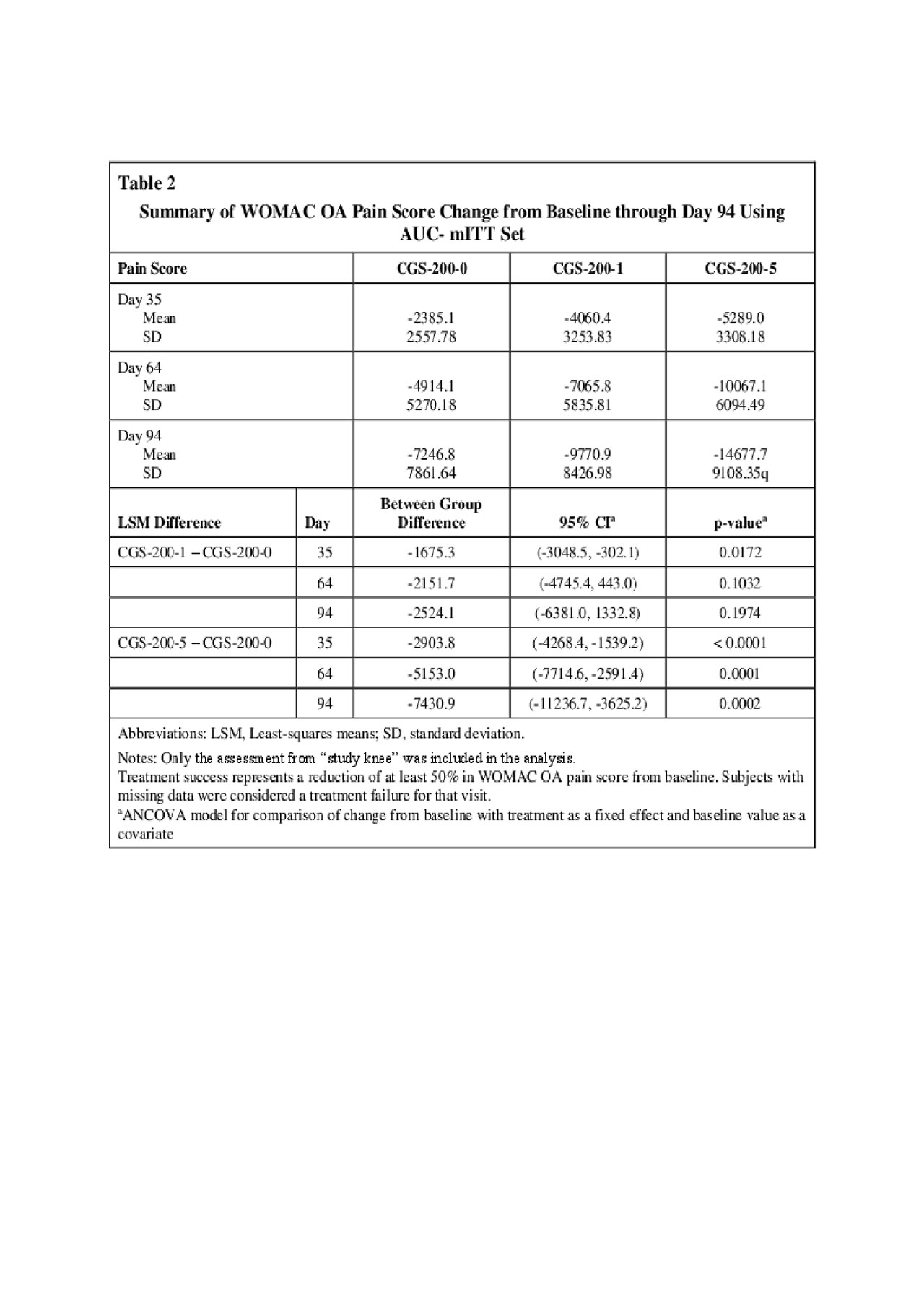
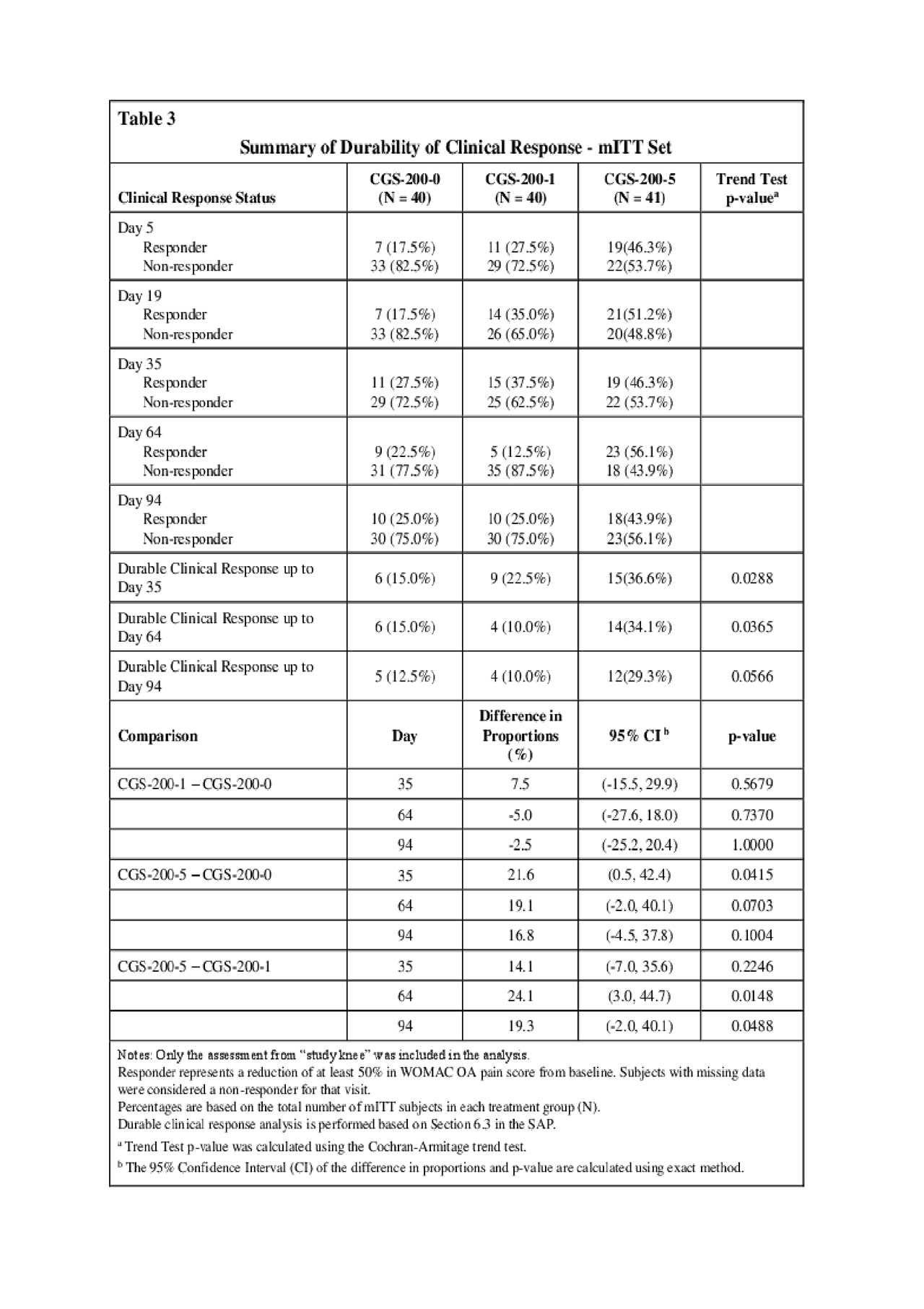
Results: CGS-200-5, but not CGS-200-1, met the primary Day 35 OAKP WOMAC pain efficacy endpoint compared to vehicle (p = 0.020, mITT population) and post-hoc analysis showed statistical separation from CGS-200-0 (Vehicle) on Days 64 (p = 0.0001) and 94 (p = 0.0111) (Table 1). Post-hoc analysis showed a statistical difference in WOMAC OA total scores between both treatment groups and Vehicle at Days 35, 64 and 94 (Table 2). The proportion of subjects with a durable clinical response (WOMAC pain reductions > 50% on all study day visits minus one) was higher in the CGS-200-5 group at all visits (Table 3). A slightly larger percentage of CGS-200-5 patients reported > 1 TEAEs (all were mild or moderate in severity) compared to the CGS-200-0 or CGS-200-1 arms, with little difference between groups in the types of TEAEs. Application site pain (mostly mild to moderate) was observed in all treatment groups, with a higher proportion of subjects in the CGS-200-5 group experiencing pain than the CGS-200-5 group. The mean tolerability AUCs in both active treatment groups decreased with each consecutive dosing day.
Conclusion: CGS-200-5 was well-tolerated and efficacious following application to both knees for 60 minutes on 4 consecutive days. Due to the significant efficacy and good safety and tolerability observed in this study, further clinical development of CGS-200-5 is warranted.
To cite this abstract in AMA style:
Billard M, Todhunter J, Fleming M, Warneke T, Qiu Y, Ly N, Aronstein W, Moore W. A Phase 2 Double-Blind Clinical Trial to Examine the Comparative Effects on Osteoarthritic Knee Pain of CGS-200-1 (1% Capsaicin Topical Liquid), CGS-200-5 (5% Capsaicin Topical Liquid), and CGS-200-0 (Vehicle, No Capsaicin) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-phase-2-double-blind-clinical-trial-to-examine-the-comparative-effects-on-osteoarthritic-knee-pain-of-cgs-200-1-1-capsaicin-topical-liquid-cgs-200-5-5-capsaicin-topical-liquid-and-cgs-200-0-v/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-double-blind-clinical-trial-to-examine-the-comparative-effects-on-osteoarthritic-knee-pain-of-cgs-200-1-1-capsaicin-topical-liquid-cgs-200-5-5-capsaicin-topical-liquid-and-cgs-200-0-v/