Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 9:00AM-9:15AM
Background/Purpose: Regulatory T cells (Tregs) modulate inflammation, maintain self-tolerance, promote tissue repair, and hold promise as a versatile therapeutic. Autologous polyclonal Tregs have a favorable safety profile but limited efficacy in autoimmunity.
Engineered Tregs offer a novel approach by introducing a chimeric antigen receptor (CAR) specific for tissue- or disease-associated antigens. SBT777101 is an autologous Treg product transduced with a CAR specific for citrullinated proteins (CitP), which are present in the inflamed synovium of rheumatoid arthritis (RA) patients. Unlike cytotoxic CAR-T therapies, SBT777101 may restore balance to the immune system without target cell destruction. Potential mechanisms of action include direct and bystander suppression of antigen presenting cells and effector T cells, IL-2 consumption, and immunomodulatory cytokine production, promoting immune homeostasis and tissue repair.
Regulate-RA is an ongoing first-in-human, phase 1 study evaluating the safety and tolerability of SBT777101 in patients with refractory RA. Exploratory endpoints include assessment of SBT777101’s pharmacokinetic (PK) profile, pharmacodynamic (PD) parameters, mechanism of action (MOA), and potential efficacy.
Methods: Adults aged 18-70 with active RA (Disease Activity Score (DAS28-CRP) ≥ 3.2) and failure of ≥3 prior classes of biologic or targeted synthetic DMARDs are eligible.
SBT777101 is prepared by transduction of autologous Tregs with a CitP-specific CAR expressing vector followed by ex vivo expansion and is administered intravenously as a single dose in a 3-cohort, 3+3 dose-escalation design, with no preparative lymphodepletion. Participants are monitored for adverse events and clinical responses through 48 weeks. PK, PD, and MOA are assessed in blood and synovial biopsies.
Results: As of August 2025, six participants have received SBT777101 (table 1). Infusions were well-tolerated, without cytokine release syndrome, neurotoxicity, or dose limiting toxicities.
Across both dose levels, 4/6 participants (67%) had a ≥ 50% reduction from baseline swollen and tender joint counts by week 4, and 4/6 (67%) had a reduction of DAS28-CRP score by ≥ 2 (figure 1). Participants in Cohort 2, who received a higher dose of SBT777101, showed a more rapid onset and consistent reduction in joint count compared to Cohort 1. All participants in Cohort 2 showed clinically meaningful improvements in disease activity by week 4 with continued reduction in disease activity for as long as 18 weeks. Additionally, all post-infusion biopsies collected to date demonstrated decreases in inflammatory score (Figure 2). SBT777101 cells can be transiently detected in blood within the first month following infusion.
Clinical assessments and biomarker analyses are ongoing to assess changes in disease activity, systemic inflammation and Treg activity; additional participants are being enrolled.
Conclusion: In this ongoing phase 1 study, Cit-P–targeted CAR-Tregs demonstrate a favorable early safety profile, with preliminary clinical and mechanistic evidence of therapeutic activity in highly refractory RA participants.
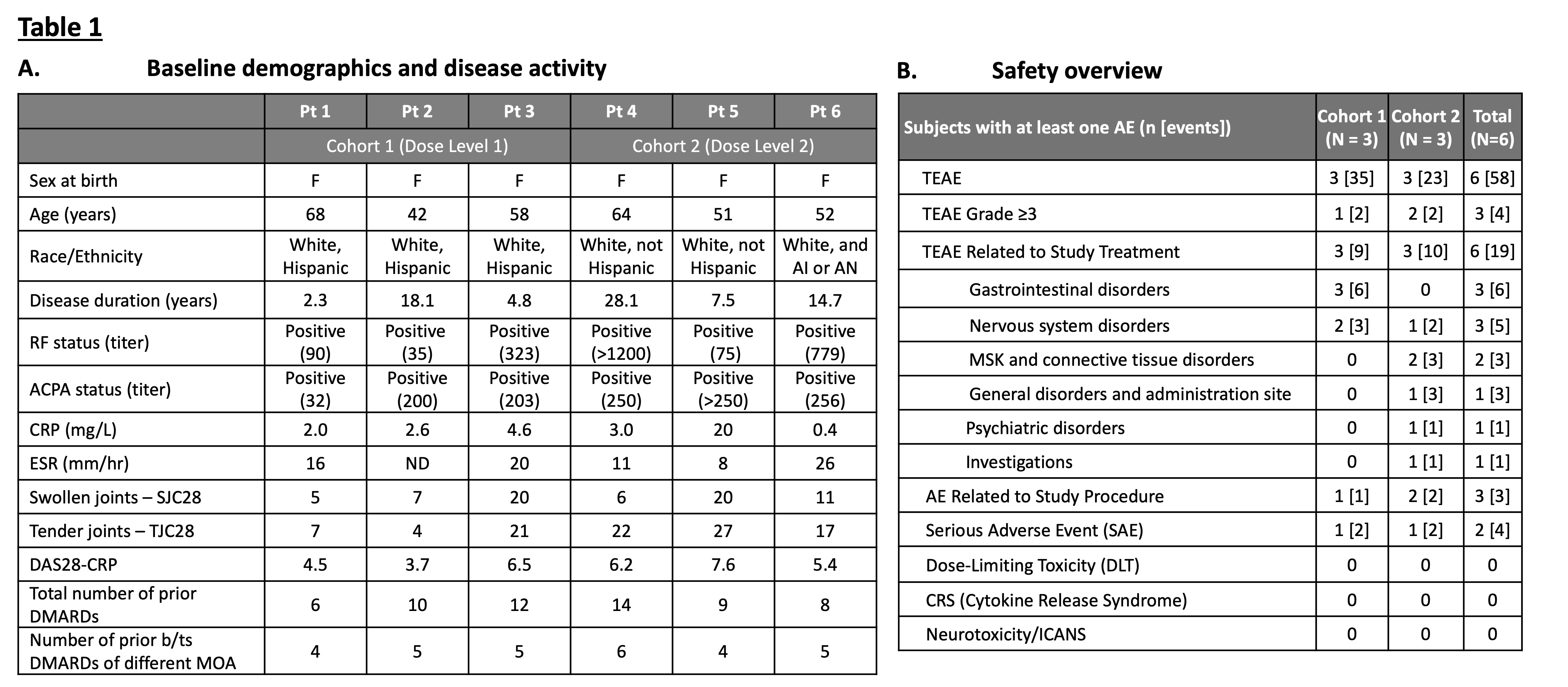 Table 1: Overview of (A) patient demographics and disease activity at screening for cohorts 1 and 2, and (B) safety events to date. AI or AN: American Indian or Alaska native; TEAE: Treatment-Emergent Adverse Event; MSK: Musculoskeletal; ICANS: Immune effector cell-associated neurotoxicity syndrome; N: the number of subjects in the Safety Evaluable Set; n: the number of subjects in each category. Data cut 8/15/2025
Table 1: Overview of (A) patient demographics and disease activity at screening for cohorts 1 and 2, and (B) safety events to date. AI or AN: American Indian or Alaska native; TEAE: Treatment-Emergent Adverse Event; MSK: Musculoskeletal; ICANS: Immune effector cell-associated neurotoxicity syndrome; N: the number of subjects in the Safety Evaluable Set; n: the number of subjects in each category. Data cut 8/15/2025
.jpg) Figure 1: Preliminary efficacy of SBT777101. (A) Cohort 1 showed modest improvement in disease activity within 4 weeks of infusion, but this was of variable duration. (B) By week 4, Cohort 2 showed more consistent improvements of both tender and swollen joint counts, as well as DAS28-CRP. SJC: swollen joint count; TJC: tender joint count; DAS: Disease Activity Score.
Figure 1: Preliminary efficacy of SBT777101. (A) Cohort 1 showed modest improvement in disease activity within 4 weeks of infusion, but this was of variable duration. (B) By week 4, Cohort 2 showed more consistent improvements of both tender and swollen joint counts, as well as DAS28-CRP. SJC: swollen joint count; TJC: tender joint count; DAS: Disease Activity Score.
.jpg) Figure 2: Reduced inflammation in post-treatment synovial biopsies: 20X images A) show pre- and B) post-treatment biopsies on the indicated joint with the patient number color represented in the dot-plot C) of Krenn inflammatory score as assessed by an independent, blinded, pathologist. Two participants only provided pre-treatment biopsies and are only shown in the plot.
Figure 2: Reduced inflammation in post-treatment synovial biopsies: 20X images A) show pre- and B) post-treatment biopsies on the indicated joint with the patient number color represented in the dot-plot C) of Krenn inflammatory score as assessed by an independent, blinded, pathologist. Two participants only provided pre-treatment biopsies and are only shown in the plot.
To cite this abstract in AMA style:
Kohler M, Arai S, Aslam F, Challener G, Frigault M, Griffith M, Katsumoto T, Massarotti E, Moreland L, Rosenthal A, Sparks J, Yinh J, Baxter S, Bitton A, Dubovsky J, Yuan V, Jensen M, Clauw A, Furman G, Gyurko R, Hall M, McIntyre A, Seifert J, Stainton E, Blake M, Fox-Bosetti S, Lebrec H, Pace A, Xiao Y, Wang M, Arron J, Bluestone J. A Phase 1 Study of Autologous CAR-Treg Cells in Refractory Rheumatoid Arthritis: Interim Report of Safety and Efficacy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-phase-1-study-of-autologous-car-treg-cells-in-refractory-rheumatoid-arthritis-interim-report-of-safety-and-efficacy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-1-study-of-autologous-car-treg-cells-in-refractory-rheumatoid-arthritis-interim-report-of-safety-and-efficacy/
