Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: For patients with refractory chronic gout, i.e., those who have frequent gout flares and/or persistent tophi despite treatment with the highest tolerable doses of oral urate-lowering therapies, pegloticase is often prescribed. In many such patients, pegloticase, a porcine-like uricase conjugated to 10-kDa methoxy-poly(ethylene glycol) (PEG), is very effective, especially when co-administered with methoxtrexate, but it is expensive, involves biweekly intravenous infusions, and may trigger serious allergic reactions. In efforts to mitigate those disadvantages, we have developed a different PEG-uricase, called PU5 (or PJ003), which is stable in vivo, has favorable pharmacodynamics (PD) and low immunogenicity and can be injected intramuscularly (IM).
Methods: A porcine-like uricase was synthesized by recombinant expression in Escherichia coli. It was PEGylated by reaction with an N-hydroxysuccinimide propionate derivative of 5-kDa PEG at a high pH. The resulting conjugates were purified chromatographically. The protein concentration was measured by the Lowry method and uricolytic activity was measured using UV absorbance. An average of 11 lysine side-chains were modified per subunit of uricase, assessed using SEC-HPLC, with detection by a combination of UV absorbance and refractive index. The primary sites of modification were identified by peptide mapping, following cleavage of uricase and PU5 by Lys-C and/or Trypsin. Pre-clinical studies of PU5 were performed using chronic hyperuricemia models in Sprague-Dawley (SD) rats and Beagle dogs. The Phase I clinical trial was a single-center, randomized, double-blind, placebo-controlled, single dose, and dose escalation trial. Healthy subjects were enrolled and the tolerability, pharmacokinetics (PK), PD and immunogenicity were assessed after one IM injection.
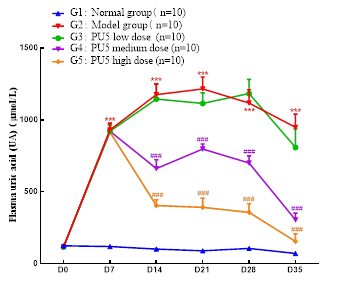
Results: (1) Figure 1 shows the key PD results obtained after an IM injection of PU5 in SD rats:
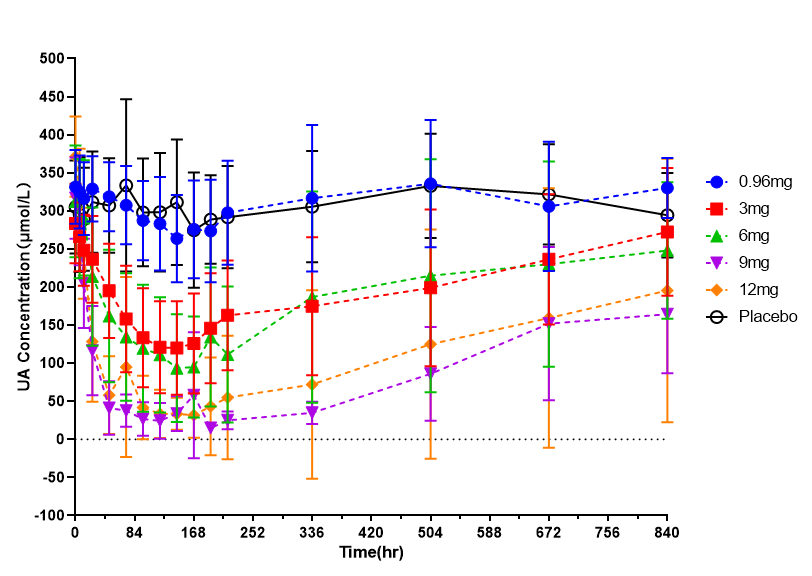
(2)Figure 2 shows the key results from the Phase I clinical trial; Intramuscular administration of the following doses: 0.96, 3, 6, 9, and 12 mg, resulted in good safety and low immunogenicity. There were 25 male and 5 female trial subjects with a mean body weight of 67 kg (range of 54 to 88 kg) and a mean age of 31 years (range of 22 to 45 years). The magnitude of the decrease in plasma uric acid (PUA) increased with the dose of PU5 and the duration of decreased PUA was positively correlated with the dose. Following a single dose of PU5 (6 – 12 mg), the PUA remained below 200 µmol/L for at least 336 hours. The time to maximal reduction of PUA (Tmax) was about 120-180 hours.
Conclusion: The Phase I clinical trial data indicated that PU5 is safe in healthy humans and has promising PK/PD and immunologic characteristics. More detailed information about PU5, including its amino acid sequence, preparation and characterization, pre-clinical PK/PD data, and Phase I clinical data, will be presented at the meeting.
To cite this abstract in AMA style:
Fan F, Liu R, Wang Y. A PEGylated Mammalian Uricase Suitable for Intramuscular Administration to Patients with Refractory Chronic Gout [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-pegylated-mammalian-uricase-suitable-for-intramuscular-administration-to-patients-with-refractory-chronic-gout/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pegylated-mammalian-uricase-suitable-for-intramuscular-administration-to-patients-with-refractory-chronic-gout/