Session Information
Date: Monday, November 11, 2019
Title: RA – Animal Models Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is a systemic inflammatory disease that manifests in a chronic and debilitating polyarthritis. Although the etiology of RA is unknown pathogenic autoreactive T cells and autoantibodies including anti-CCP and anti- malondialdehyde-acetaldehyde (MAA) are thought to contribute to disease pathogenesis. Our recent studies identified the microbial sensor Nod2 as a critical genetic determinant in controlling the Th17-response and arthritis severity in SKG mice (Napier RJ et al. J Immunol 2018). However, whether Nod2 also contributes to autoantibody production in SKG mice is unknown. The purpose of this study was to extend our understanding of the protective mechanisms by which Nod2 controls arthritis in SKG mice to humoral immunity.
Methods: Balb/c, SKG and Nod2-/-SKG mice (6-8 wk age) were housed under specific pathogen-free conditions. Arthritis was induced by i.p. injection of 1.5 mg zymosan (Sigma-Aldrich) vs. saline (sham control). Serum IgG anti-CCP and anti-MAA antibodies were measured by ELISA 8 weeks later. Clinical arthritis for each paw was graded (0–4) in masked fashion. T cells (CD4+ and CD8+) were isolated from joint synovial fluid and evaluated (#T cells/ml) by flow cytometry.
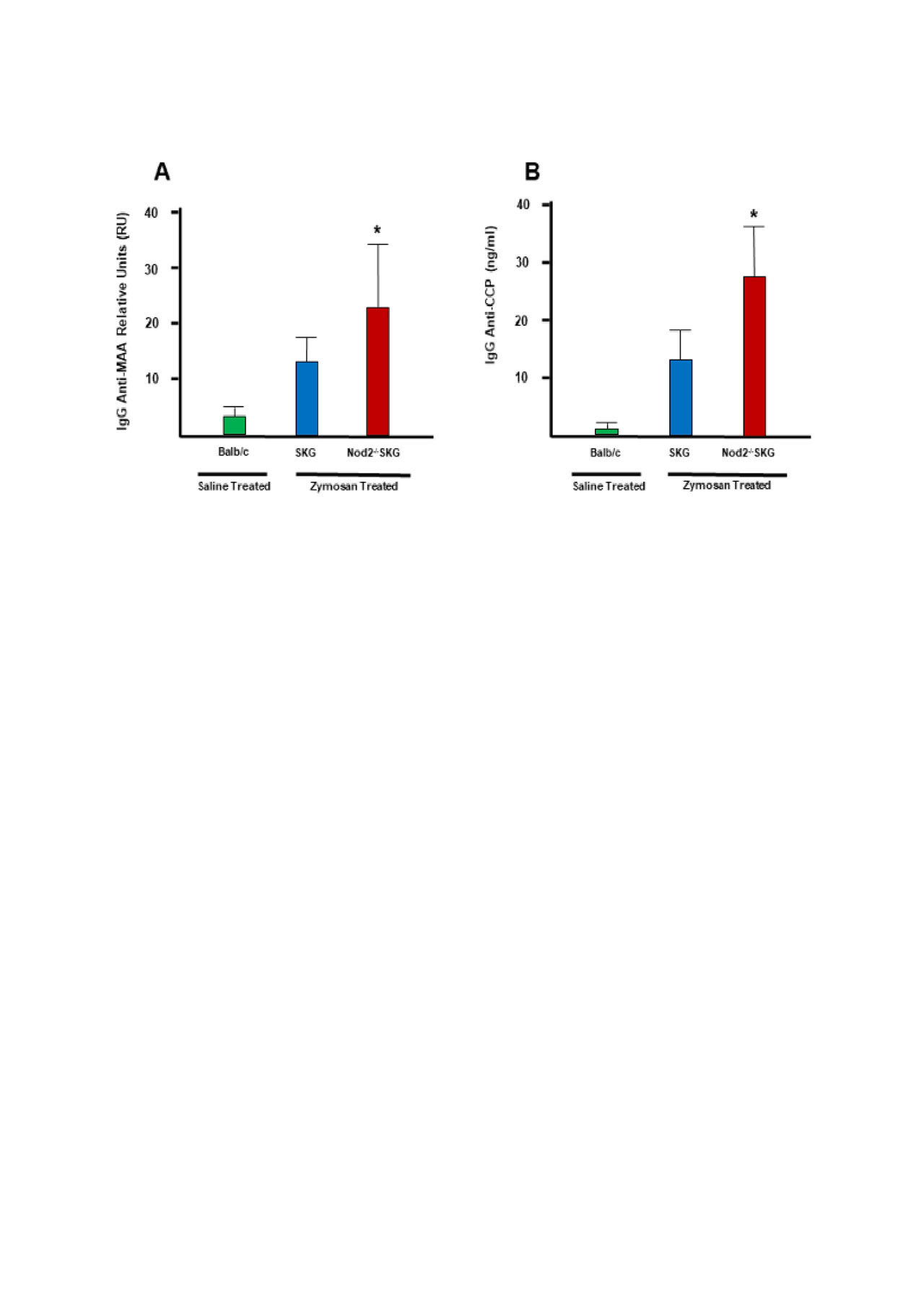
Results: Nod2-/-SKG mice had significantly increased concentrations of serum anti-CCP and anti-MAA autoantibodies at 8 weeks post zymosan exposure compared to zymosan-exposed SKG (p = 0.0111 for CCP; and, p = 0.0223 for MAA) and sham treated Balb/c mice (p = 0.0371 for CCP; and, p = 0.0430 for MAA), the latter group demonstrating only negligible autoantibody reactivity. While there were increased concentrations of anti-CCP and anti-MAA antibodies in SKG mice exposed to zymosan compared to saline exposed Balb/c mice, they were not significantly higher (p = 0.080 for CCP; and, p = 0.111 for MAA). Comparisons with previously reported data (Napier et al.) showed serum anti-CCP and anti-MAA concentrations were increased in conjunction with arthritis scores in SKG and Nod2-/-SKG mice stimulated with zymosan. Likewise, we observed similar increases anti-CCP and anti-MAA concentrations that were associated with the total number of CD4+ T cells present in the synovial fluid collected from SKG and Nod2-/-SKG mice stimulated with zymosan.
Conclusion: These data suggest a previously unprecedented role for Nod2 in suppressing autoreactive B cell responses in the SKG arthritis model. Increased autoantibody concentrations correlated with increased synovial T cell responses and more severe arthritis. Understanding the mechanisms and consequences of enhanced autoantibody production in Nod2-deficient SKG mice will contribute to our understanding of the pathophysiology of RA. Moreover, arthritis resulting from Nod2 deficiency in SKG mice renders a disease model that is characterized by enhancements in both T cell responses and humoral immunity, suggesting that the Nod2-/-SKG mice might provide a highly relevant system for modeling RA.
To cite this abstract in AMA style:
Napier R, Vance E, O'Dell J, Poole J, England B, Duryee M, Mikuls T, Rosenzweig H, Thiele G. A Novel Role for Nod2 in Controlling Autoantibody Production and Arthritis in SKG Mice [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-novel-role-for-nod2-in-controlling-autoantibody-production-and-arthritis-in-skg-mice/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-role-for-nod2-in-controlling-autoantibody-production-and-arthritis-in-skg-mice/