Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tyrosine kinase 2 (TYK2), a member of the Janus kinase (JAK) family, plays a key role in several inflammatory diseases. Orthosteric, small molecule inhibitors of TYK2 can also bind other JAK family members (JAK1, 2 and/or 3), which complicates their therapeutic profile. Allosteric inhibitors, which target the pseudokinase (JH2) domain of TYK2, show greatly improved selectivity, providing efficacy in immune disorders without concurrent JAK inhibition. Although there are allosteric TYK2 inhibitors in development, efficacy matching that of the anti-IL-23 biologics has not yet been achieved, and there remains a need for a more efficacious oral agent. Here we describe a new drug candidate, ATMW-DC, a novel, potent, and selective allosteric inhibitor of human TYK2, discovered using the AtomNet® artificial intelligence-enabled drug discovery platform. The objective of this study was to determine the biochemical and cellular potency and selectivity of ATMW-DC and to evaluate its efficacy in mouse models of psoriasis.
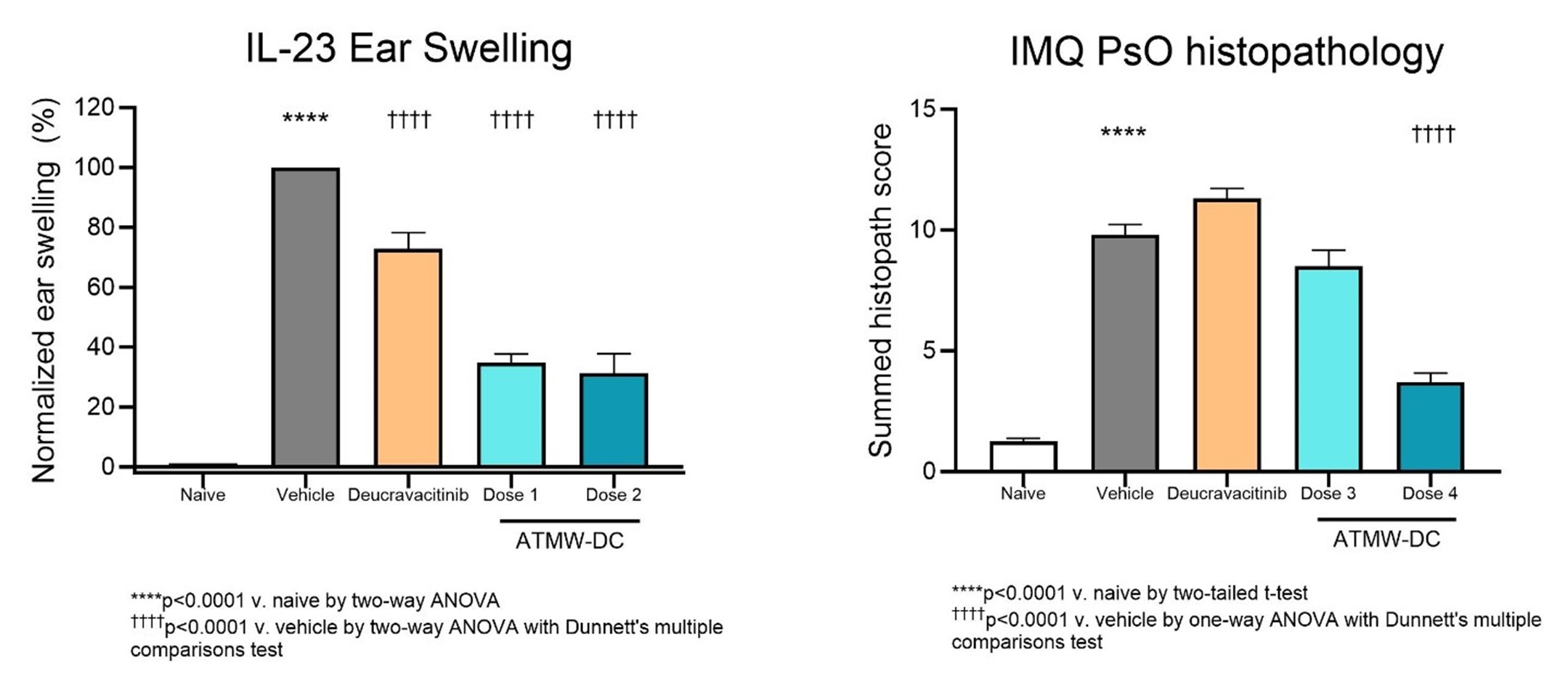
Methods: The potency and selectivity of ATMW-DC were determined in biochemical competition assays and cellular cytokine-induced STAT (signal transducer and activator of transcription) phosphorylation assays. ATMW-DC was also evaluated in a mouse pharmacodynamic (PD) model, where ear swelling and IL-17A levels were measured after 4 days of subcutaneous IL-23 injection ± oral ATMW-DC (Doses 1 and 2). An acute model of psoriasis, based on imiquimod (IMQ)-induced skin inflammation, was used to evaluate efficacy of ATMW-DC (Doses 3 and 4) via histological and clinical assessments and disease-relevant cytokine/chemokine levels in the skin. ATMW-DC Doses 1 and 3 were selected to target the human whole blood IC90 while Doses 2 and 4 targeted the IC99. In both studies, deucravacitinib was used as a positive control (at a dose targeting clinically relevant exposures).
Results: ATMW-DC exhibited potency (TYK2-JH2 IC50= 12pM) and selectivity for TYK2-JH2 (≥350-fold over JAK1, 2 or 3) in biochemical binding assays. Against a kinome panel, ATMW-DC demonstrated a high degree of selectivity with a partition index (PTYK2-JH2) of 0.98. In cellular cytokine assays, ATMW-DC inhibited IL-12-induced pSTAT4 (IC50= 18nM) with >460-fold selectivity over JAK1/2-signaling cytokines (IL-6/pSTAT3 IC50 >8.5μM, GM-CSF/pSTAT5 IC50 >8.3μM). In vivo, ATMW-DC showed dose-dependent inhibition of ear swelling (65-69%) and IL-17A cytokine levels (11-73%) in an IL-23-driven PD model. In the IMQ-induced mouse model of psoriasis, oral administration of ATMW-DC resulted in significant inhibition of inflammation, as measured by daily PASI summed scores (p < 0.001), spleen weight (p < 0.001), histopathology scores (p < 0.001), and skin cytokine/chemokine levels (p < 0.001 inhibition of IL-17A, GM-CSF, and TNF and p< 0.05 for CCL3).
Conclusion: ATMW-DC is a novel, oral, potent and selective allosteric inhibitor of TYK2. The in vitro profile and in vivo efficacy of ATMW-DC (in both the IL-23 PD and IMQ mouse models) support its further development for the treatment of autoimmune and autoinflammatory diseases driven by TYK2-mediated pathways.
To cite this abstract in AMA style:
Hussein R, Tsuruda P, Mortezaei S, Ferdyan N, Wegerski C, Srinivasan K, Hirst G, Mozaffarian N. A Novel, Oral, Allosteric Inhibitor of Tyrosine Kinase 2 (TYK2) Demonstrates In Vitro Potency, Selectivity, and In Vivo Efficacy in Mouse Models of Psoriasis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-novel-oral-allosteric-inhibitor-of-tyrosine-kinase-2-tyk2-demonstrates-in-vitro-potency-selectivity-and-in-vivo-efficacy-in-mouse-models-of-psoriasis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-oral-allosteric-inhibitor-of-tyrosine-kinase-2-tyk2-demonstrates-in-vitro-potency-selectivity-and-in-vivo-efficacy-in-mouse-models-of-psoriasis/