Session Information
Date: Sunday, October 26, 2025
Title: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
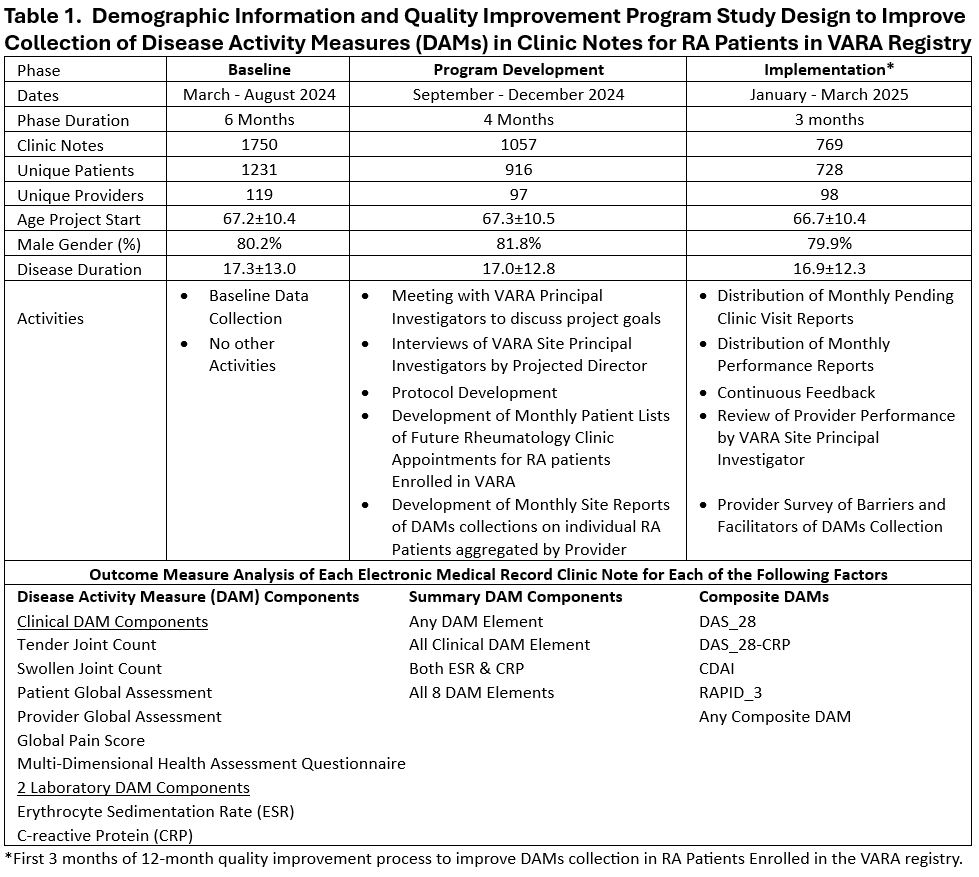
Background/Purpose: Clinical guidelines recommend the use of disease activity measures (DAMs) in rheumatoid arthritis (RA) management. Measurement of DAMs is also a critical component of clinical and epidemiologic research in RA. The Veterans Affairs RA (VARA) registry is a prospective cohort study of Veterans with RA that is active at 16 VA Medical Centers. A VARA goal is to systematically collect and document eight DAM components for participants at each in-person clinic visit (Table1). A multimodal intervention was initiated to enhance DAMs collection. This report details preliminary results following three months of implementation of this planned 12-month program.
Methods: This intervention involved three phases: 1) baseline; 2) development: and 3) implementation (Table 1). Key activities included qualitative interviews with site investigators to confirm availability of electronic health record (EHR) tools for DAM collection, provision of additional EHR tools if needed, development of monthly just-in-time lists of VARA participant appointments, and monthly provider-specific feedback on DAMs collection performance and comparison to VARA registry overall performance. Verification and retrieval of documented DAM components in the EHR was accomplished through data extraction of note text form the VA Corporate Data Warehouse. Performance at each encounter, by provider and by VARA site over the 3-month implementation phase was compared to baseline performance using chi square testing.
Results: Between March 2024 through March 2025, 3,576 in-person rheumatology clinic visits occurred in 1,436 RA patients enrolled in VARA who were seen by 135 unique providers. The distribution of these visits and basic demographic information on these patients during the baseline, development, and implementation phases are listed (Table 1). Compared to baseline values, there was a significant increase in the documentation of individual DAM components, combined DAM components, and composite DAMs during the implementation phase with increases ranging from 5.8% to 10.4% (Table 2). Analyses limited to the 44 providers completing ≥5 clinical notes during both the baseline and implementation phases demonstrated an increase in the number of providers successfully documenting DAMs in >50% of encounters (Table 3). A separate analysis of DAMs collection by all providers at each VARA site also showed improvement in the number of VARA sites successfully documenting DAMs (Table 3).
Conclusion: This multimodal intervention increased DAM collection and documentation in clinical notes, expanded the number of providers consistently collecting DAMs, and enhanced the performance at VARA registry sites during an initial 3-month intervention phase. Ongoing monitoring will determine if additional performance improvement occurs through sustained intervention over a 12-month follow-up. This work demonstrates that collaborative actions in a multi-center registry can improve both the quantity and quality of high-yield clinical data collected to facilitate patient care and research. These methods may be applied in additional clinical settings to improve real-world collection of DAMs.
To cite this abstract in AMA style:
Cannon G, Wallace B, Lazaro D, Schwab P, Monach P, Shah A, Kerr G, Reimold A, Baker J, Kunkel G, Wysham K, Caplan L, Richards J, Lenert A, Jones A, Mikuls T, Danila M, England B, Sauer B, Rojas J, Smith I. A Multimodal Intervention Improves the Quantity and Quality of Disease Activity Measures Collection in a Multi-Centered National Rheumatoid Arthritis Network [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-multimodal-intervention-improves-the-quantity-and-quality-of-disease-activity-measures-collection-in-a-multi-centered-national-rheumatoid-arthritis-network/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multimodal-intervention-improves-the-quantity-and-quality-of-disease-activity-measures-collection-in-a-multi-centered-national-rheumatoid-arthritis-network/

.jpg)
.jpg)