Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a complex systemic inflammatory autoimmune disease with a high incidence, primarily affecting the joints. However, it currently lacks definitive biomarkers for clinical onset, disease severity, and treatment responses. Recently, the plasma proteomics has emerged as a powerful and promising tool for assessing human health and disease conditions (Nature 2023, 164-172), garnering increasing attention. Despite some advancements in the proteomic studies of RA, there remains a compelling need for longitudinal cohort studies to deepen our understanding. Our study aims to fill this knowledge gap.
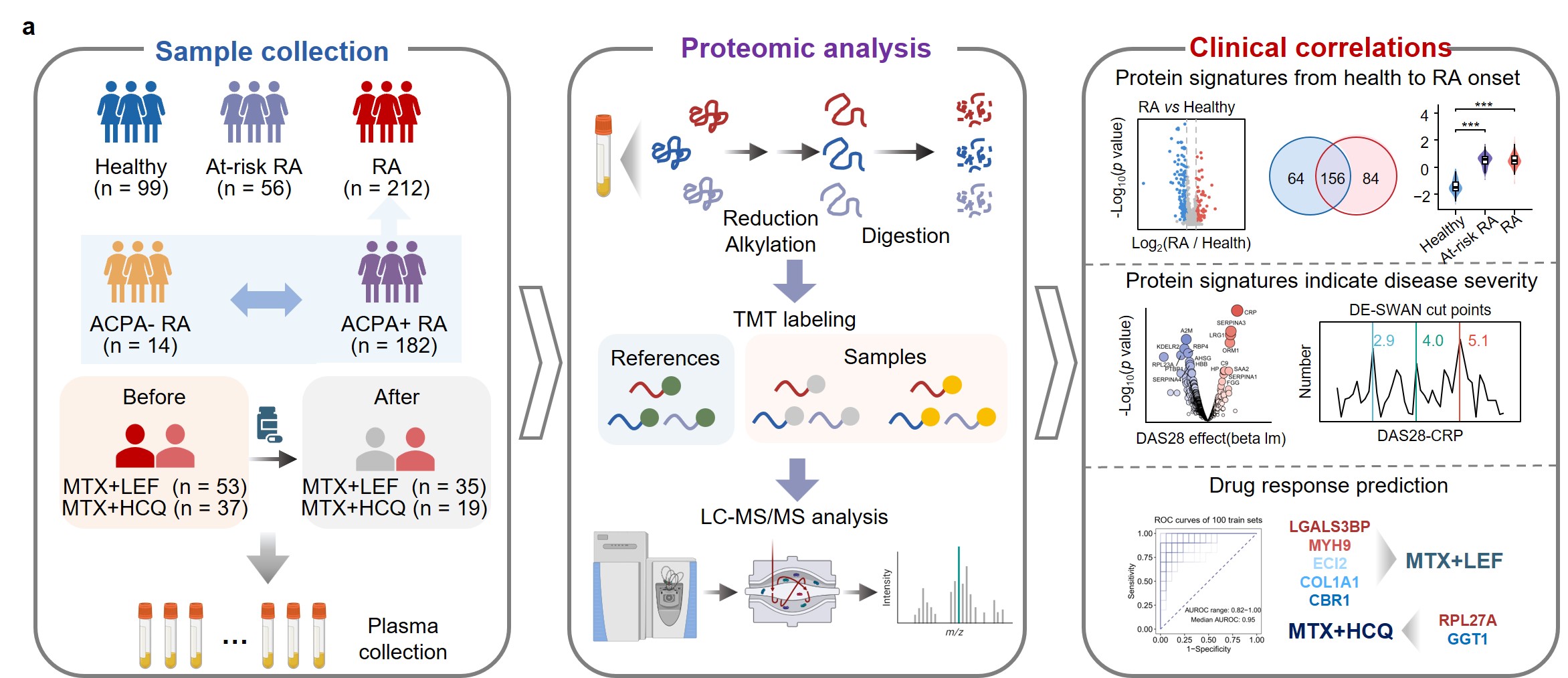
Methods: We investigated a longitudinal cohort comprising 212 RA patients, including 204 with one or two follow-ups, along with 56 at-risk RA individuals and 99 healthy controls, to elucidate plasma protein biomarkers predating clinical onset, disease severity and treatment response. We developed a tandem mass tag (TMT)-based proteomic strategy adding carrier proteins to amplify the signal of low-abundance plasma proteins. In this approach, there’s no requirement to remove high-abundance proteins, enabling the identification of plasma proteins with high throughput using just a single mass spectrometry (MS) injection.
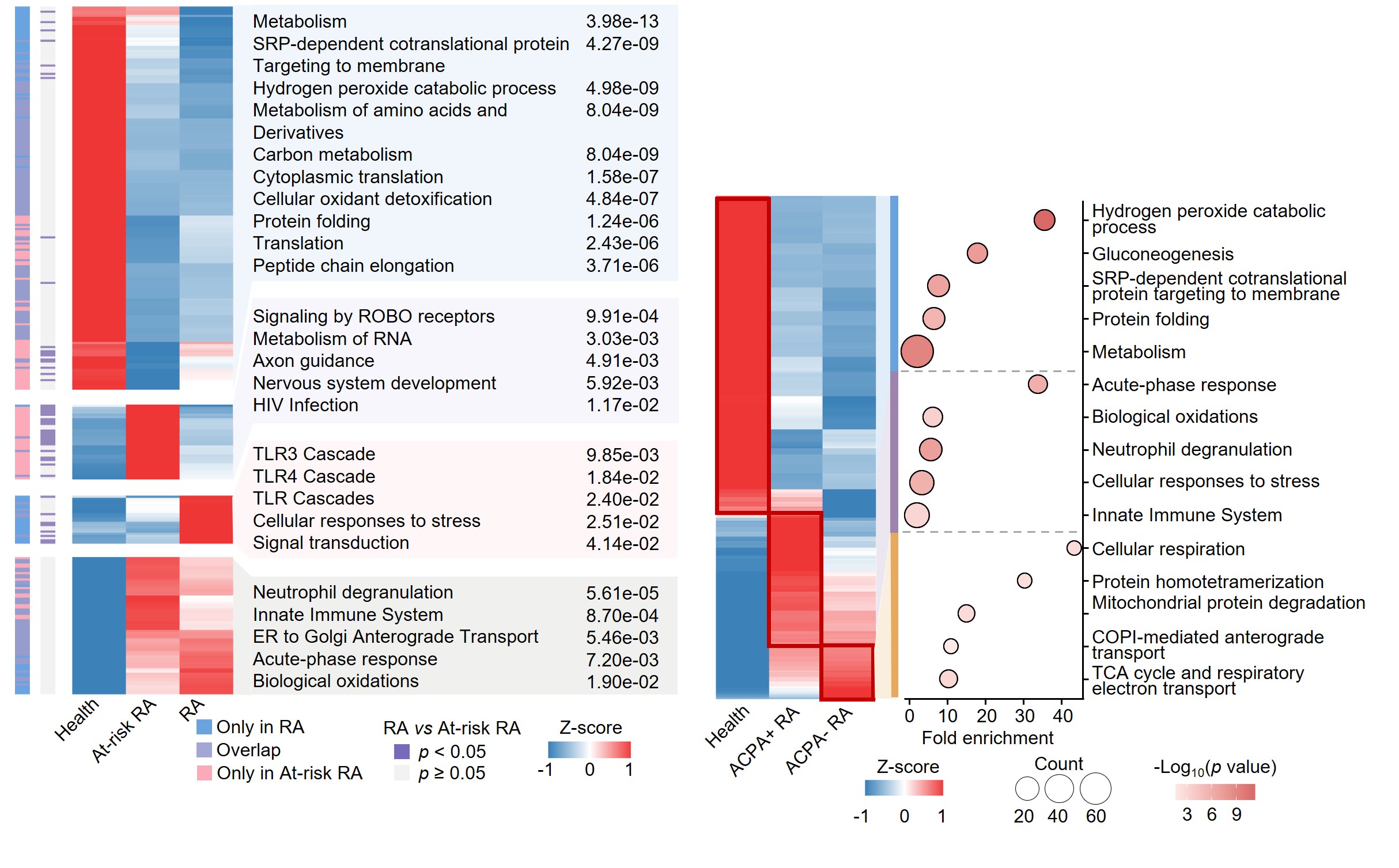
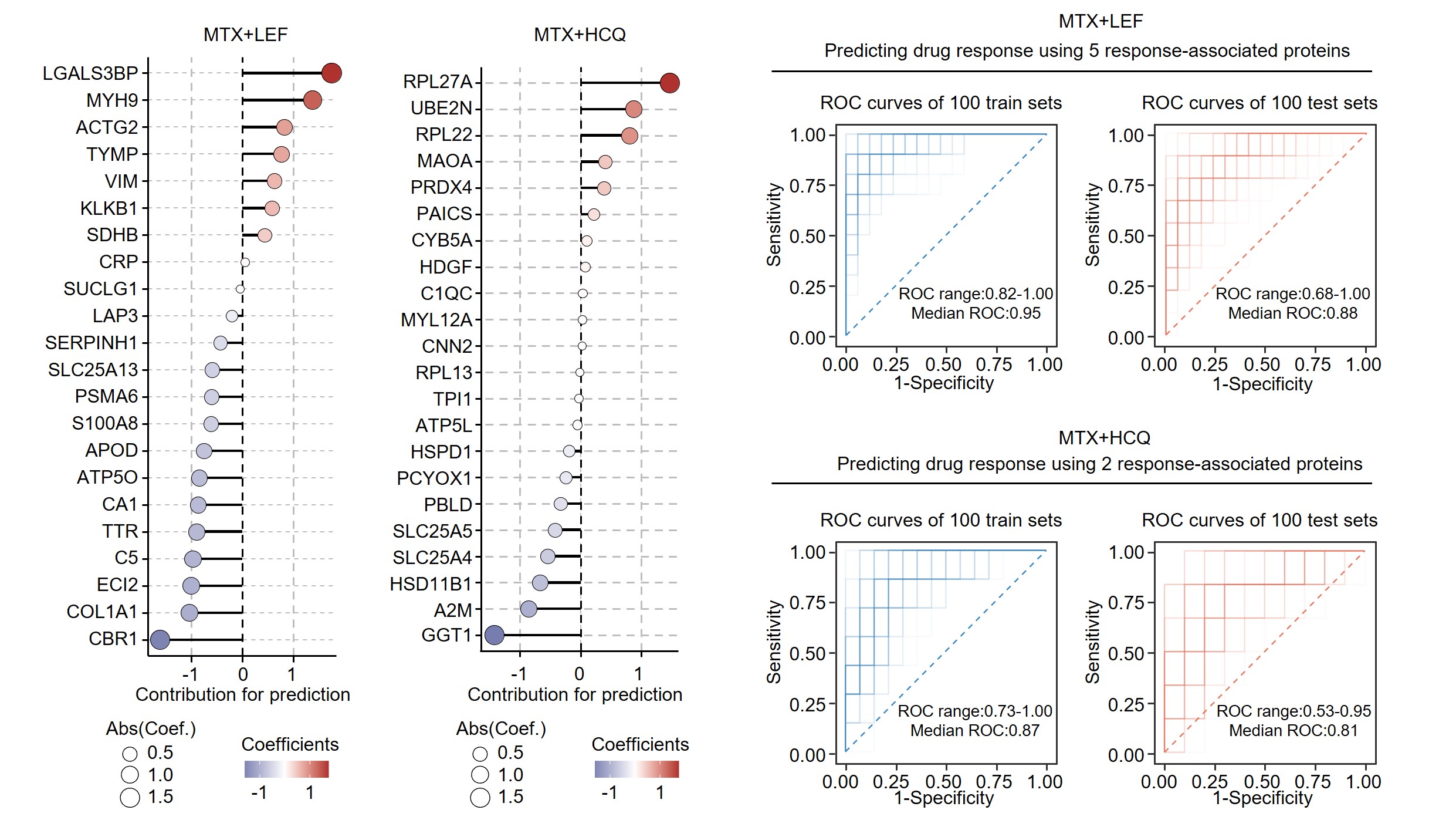
Results: Proteins related to neutrophil degranulation and acute phase responses were upregulated in RA and at-risk individuals, while proteins involved in protein processing and metabolism were downregulated. Proteins specifically elevated in at-risk RA individuals were linked to nervous system development and RNA metabolism. ACPA-negative RA patients exhibited increased TCA cycle and cellular respiration proteins, while ACPA-positive RA patients showed heightened acute phase responses and neutrophil degranulation. Alterations in plasma protein levels exhibited correlations with disease activity, peaking at DAS28 scores of 2.9, 4.0, and 5.1, respectively. Our findings revealed that the combination of methotrexate (MTX) and leflunomide (LEF) effectively modulated pro-inflammatory pathways, while MTX in combination with hydroxychloroquine (HCQ) significantly impacted energy metabolism. Utilizing machine learning models, we accurately predicted treatment responses, with MTX+LEF achieving average ROC scores of 0.95 for training set and 0.88 for testing set, and MTX+HCQ yielding average ROC scores of 0.87 for training set and 0.81 for testing set.
Conclusion: Our study delineated the characteristic molecular profiles of RA, at risk and health groups, uncovering potential therapeutic targets for interventions in the preclinical or early stages of RA, as well as in seronegative RA. We explored proteins undergoing linear and nonlinear changes with DAS28, identifying fluctuation peaks at scores of 2.9, 4.0, and 5.1. Proteomics research differentiated between responders and non-responders to MTX+LEF and MTX+HCQ treatments, facilitating the development of predictive models and enhancing the understanding of the mechanisms behind suboptimal responses to csDMARD therapy.
To cite this abstract in AMA style:
Zhu C, He S, Liu Y, Sun R, Zhao Y. A Longitudinal Cohort Study Uncovers Plasma Protein Biomarkers Predating Clinical Onset and Treatment Response of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-longitudinal-cohort-study-uncovers-plasma-protein-biomarkers-predating-clinical-onset-and-treatment-response-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-longitudinal-cohort-study-uncovers-plasma-protein-biomarkers-predating-clinical-onset-and-treatment-response-of-rheumatoid-arthritis/