Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The mechanisms by which the gut microbiome contributes to autoimmune pathogenesis remain poorly understood. In lupus patients, more than ten-fold blooms of Ruminococcus gnavus (RG) occur during nephritis flares. We, therefore, sought to identify mechanisms promoting the gut expansion of this pathobiont and its contribution to autoimmune pathogenesis.
Methods: With cellular and serologic assays, we evaluated the effects of RG gut colonization in lupus-prone B6.Sle1.Sle2.Sle3 (TC) mice. Fecal microbiota transfers (FMT) from active and inactive TC mice, vs controls were assessed in recipient mice, along with the influence of dietary tryptophan. The growth and metabolome of RG strains were evaluated in response to tryptophan, and for the direct in vitro effects of RG on Treg cells.
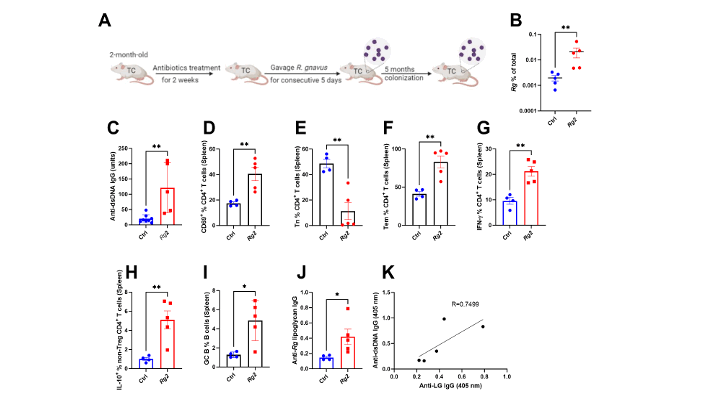
Results: After gavage, TC mice exhibited persistent colonization with only the lipoglycan (LG)-producing RG strain, which led to increased intestinal permeability and raised serum inflammatory cytokines, autoantibodies and T-cell activation. Conversely, after RG depletion Treg representation increased and inflammation subsided. Co-cultures of dendritic cells with LG-producing RG-induced Treg apoptosis. Whereas microbiota communities in TC mice naturally include RG and dietary tryptophan caused further RG expansion. Tryptophan metabolism also differed between the two strains tested. In standard medium, the lupus-associated RG strain produced less tryptamine, and less indole-3-acetaldehyde (IAAld) that in health acts through the Aryl hydrocarbon receptor (AhR) to improve intestinal barrier function. These observations suggest that the lupus-associated RG strain possesses a differential tryptophan catabolic pathway that correlates with pathogenic potential. Together, RG induced intestinal inflammation and systemic autoimmunity which was further accelerated by a high tryptophan diet.
Conclusion: Colonization with an LG-producing RG strain, akin to strains expanded in lupus patients in flare, directly contributes to Treg reduction and immune activation that drive autoimmunity. Hence, host genetics and tryptophan metabolism promote RG growth and immunoregulatory metabolite production. We therefore propose that targeted RG depletion may improve lupus outcomes.
To cite this abstract in AMA style:
Silverman G, Morel L. A Human Lupus Gut Pathobiont Accelerates Systemic Inflammation, Autoantibody Production and T Cell Dysregulation [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-human-lupus-gut-pathobiont-accelerates-systemic-inflammation-autoantibody-production-and-t-cell-dysregulation/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-human-lupus-gut-pathobiont-accelerates-systemic-inflammation-autoantibody-production-and-t-cell-dysregulation/