Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The value of intra-articular (IA) corticosteroid injections for osteoarthritis of the knee (KOA) has recently been called into question. Variability in clinical trial design has resulted in inconsistent results from clinical studies evaluating efficacy of this commonly performed procedure. We designed a pilot and feasibility double-blind trial to evaluate the benefit of IA corticosteroid injections with monitoring of response using mobile devices at 2-week intervals.
Methods: Participants with KOA, ages 40-80, who were either receiving or found to be a candidate for ia corticosteroid injection by their physician were eligible for enrollment. Participants received a FitbitTM activity monitor and were signed up to an online platform (Way To Health). Participants were randomized after a 2-4 week run-in period to assess baseline physical activity. Participant could receive 40 mg methylprednisolone acetate plus 2 mL 1% lidocaine or 2 mL 1% lidocaine only in each affected knee. Participants were also randomized in a factorial design to receive social incentives to promote exercise (not shown here). Participants and study staff were blinded to treatment allocation and the research pharmacist prepared an opacified syringe to the physician at the time of the injection. Text messages were sent at 2-week intervals to remind participants to complete the Knee Injury and Osteoarthritis Outcome Score (KOOS). The Minimal Clinically Important Difference (MCID) for KOOS subscales ranges between 16 and 18. The primary outcome was the change in total KOOS from baseline. We compared change in KOOS across treatment groups incorporating all outcome measures over 12 weeks (2, 4, 6, 8, 10, and 12 weeks) using linear regression with generalized estimating equations. Sensitivity analyses were performed utilizing a last-observation-carried-forward (LOCF) approach.
Results: At the time of this interim analysis, 27 out of 32 planned participants (24 male) were randomized (Figure 1). Baseline characteristics are shown in Table 1. A total of 18 had completed all 12 weeks of follow-up. Participants that received corticosteroids had significantly greater improvement in overall KOOS score [B: 10.2 (2.7, 17.7) p=0.007]. This pattern was observed for all KOOS sub-scales (Figure 2), and was most prominent between weeks 4 and 10 weeks of follow-up. Three adverse events were reported during the trial; none were considered related to the intervention. The effect was robust with imputation of missing values in sensitivity analyses using LOCF [B: 10.2 (2.8, 17.6) p=0.007]. In addition, among the 18 participants that had completed follow-up, the difference was significant at 12-weeks [B: 14.4 (0.58, 28.1) p=0.007]. The number needed to treat to achieve an improvement in pain at least as much as the MCID was 3.
Conclusion: In this pilot double-blinded randomized trial, there was a significant benefit of ia corticosteroid injections compared to lidocaine. The intervention was beneficial in terms of pain, function, and quality of life and was clinically-important in magnitude, though the effects start to diminish by week 12. This study suggests that trials evaluating benefits of IA corticosteroid should consider the importance of short-term benefits.
 Figure 1: Consort Diagram. Corticosteroid Lidocaine Only N 13 14 Age 58.4 (2.0) 63.1 (2.1) Female, N (%) 2 (15%) 1 (7%) Black 9 (69%) 11 (79%) BMI 35.5 (1.8) 31.8 (1.5) Current Smoker 2 (15%) 2 (14%) K-L grade 3 (2, 4) 4 (3, 4) Baseline KOOS 38.8 (17.2) 44.0 (14.4) Prior Injections 13 (100%) 14 (100%)
Figure 1: Consort Diagram. Corticosteroid Lidocaine Only N 13 14 Age 58.4 (2.0) 63.1 (2.1) Female, N (%) 2 (15%) 1 (7%) Black 9 (69%) 11 (79%) BMI 35.5 (1.8) 31.8 (1.5) Current Smoker 2 (15%) 2 (14%) K-L grade 3 (2, 4) 4 (3, 4) Baseline KOOS 38.8 (17.2) 44.0 (14.4) Prior Injections 13 (100%) 14 (100%)
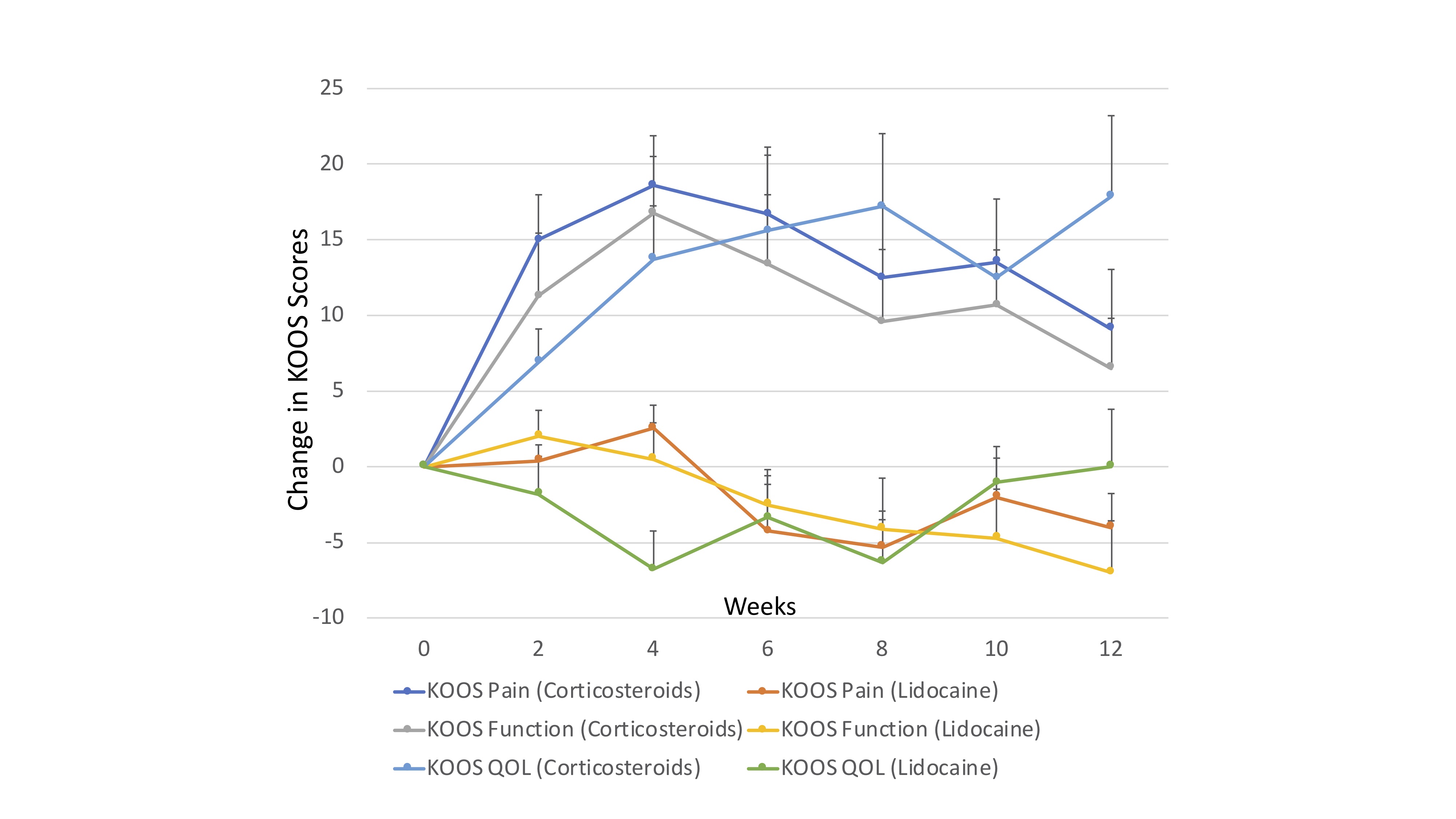 Figure 2: Mean change from baseline (SE) in KOOS scores (pain, function, and quality of life) over 12 weeks of follow-up by treatment arm.
Figure 2: Mean change from baseline (SE) in KOOS scores (pain, function, and quality of life) over 12 weeks of follow-up by treatment arm.
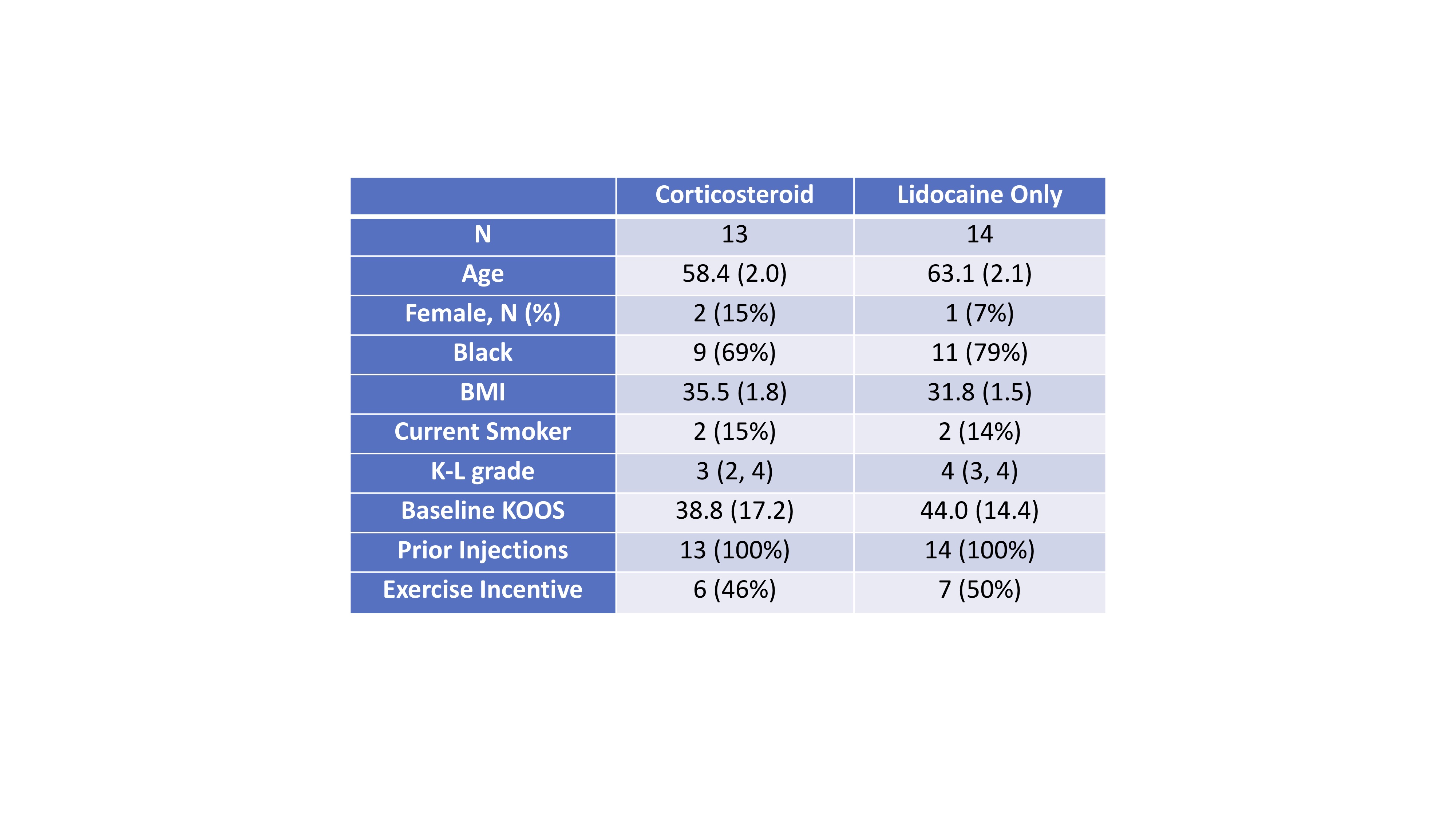 Table 1: Patient characteristics at enrollment among participants receiving corticosteroids + lidocaine v. lidocaine only.
Table 1: Patient characteristics at enrollment among participants receiving corticosteroids + lidocaine v. lidocaine only.
To cite this abstract in AMA style:
Baker J, Patel M, Neogi T, Robinson K, Ogdie A, Scanzello C. A Double-Blind Randomized Trial to Evaluate the Efficacy of Corticosteroid Injections for Osteoarthritis of the Knee Using Mobile Devices [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-double-blind-randomized-trial-to-evaluate-the-efficacy-of-corticosteroid-injections-for-osteoarthritis-of-the-knee-using-mobile-devices/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-double-blind-randomized-trial-to-evaluate-the-efficacy-of-corticosteroid-injections-for-osteoarthritis-of-the-knee-using-mobile-devices/
