Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: To demonstrate non-inferiority of the response of musculoskeletal ultrasound (MSKUS)-assessed synovitis to baricitinib, alone and plus MTX vs. etanercept plus MTX in patients with RA.
Methods: This was a 3-arm, randomized 1:1:1, open-label, parallel active-controlled, multicentre international, phase IV low-intervention clinical trial. Participants were adult patients diagnosed with RA with moderate to severe disease activity (Disease Activity Score for 28 joints ≥ 3.2) and inadequate response to MTX. Patients were randomized into one of the three arms: Arm 1 (50 patients): baricitinib in monotherapy; Arm 2 (50 patients): baricitinib + MTX; Arm 3 (50 patients): etanercept + MTX. Patients with no clinical response according to European Affiliation of Associations for Rheumatology (EULAR) response criteria at week 12 discontinued the study and received rescue treatment at investigator’s discretion. Patients underwent clinical, laboratory and MSKUS assessment at baseline, 4 weeks, 12 weeks (primary outcome), and 24 weeks. Bilateral wrist and 1st-5th metacarpophalangeal joints were graded for B-mode synovial hypertrophy, Doppler synovitis, and EULAR-Outcome Measures in Rheumatology (OMERACT) combined synovitis score. For each patient we obtained a total score for each MSKUS variable from the sum of their grades at each site. The MSKUS investigators were blinded to the assigned treatment and the clinical and laboratory data. Non-inferiority was claimed if changes in MSKUS-assessed synovitis with baricitinib (alone and combined with MTX) after 12 weeks of treatment were at least 80% of changes in the etanercept + MTX group in the same period using a non-inferiority test for two means. We present the interim results from 107 patients at week 12.
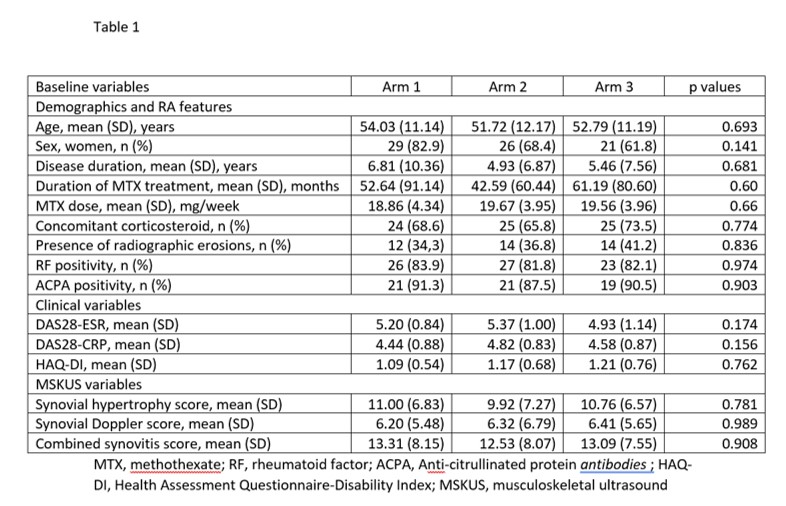
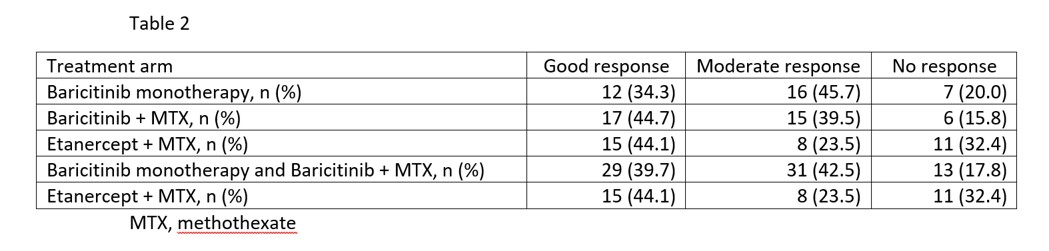
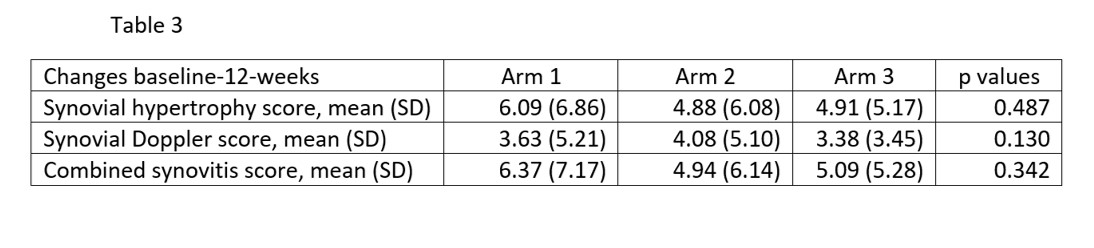
Results: 107 patients (76 female, 31 male) randomized at 13 centers were included. Of them, 33% received baricitinib in monotherapy, 36% baricitinib + MTX, and 32% etanercept + MTX. Table 1 shows demographics and baseline RA features, clinical and MSK data of patients in the 3 treatment arms. There were no significant differences in baseline variables. At week 12, EULAR response was similar in the 3 treatment arms (p=0.249) and in the baricitinib group (i.e., baricitinib in monotherapy and baricitinib + MTX) vs the etanercept + MTX group (p=0.101) (Table 2). A week 12, the mean changes in the MSKUS scores were not significantly different between the 3 treatment arms (Table 3). There were no significant differences in changes in the MSKUS scores between the baricitinib group (i.e., baricitinib monotherapy and baricitinib + MTX) vs the etanercept group or between the baricitinib monotherapy group versus the baricitinib + MTX group. The criterion of non-inferiority of baricitinib/baricitinib + MTX vs etanercept + MTX was met for synovial hypertrophy (t=2.211; p=0.015), synovial Doppler (t=2.237; p=0.014) and synovitis combined score (t=2.289; p=0.012). There were three serious adverse events (2 in the baricitinib + MTX arm, 1 in the etanercept + MTX arm) that required hospitalisation, all with favourable outcome.
Conclusion: MSKUS-determined response to baricitinib/baricitinib + MTX was non-inferior to that of etanercept + MTX.
To cite this abstract in AMA style:
NAREDO E, Olivas-Vergara O, Borges P, Recuero-Díaz S, Saraiva F, Martinho J, Costa F, Tenazinha C, Monteiro M, Melo A, Rodriguez-García A, Guillén-Astete C, Blanco-Cáceres B, Boteanu A, Mera-Varela A, Pérez Pampín E, López Golán Y, Campos Fernández C, Fragío Gíl J, gonzalez mazarío r, De Agustín J, Añez Sturchio G, Tarancon L, Vicente-rabaneda E, Castañeda S, Llorente-Cubas I, Stoenoiu M, Adrien N, Padovano I, Gouze H, Leboime A, Breban M, Uson J, Villaverde V, Steiner M, Vergara Dangond C, Paredes Romero M, Muñoz Fernández S, Pérez-García C, Meraz-Ostiz J, Finzel S, Kanne A, Mediero A, Herencia C, Herrero-Beaumont G, Largo R. A 3-arm, Randomized, Open-label, Parallel Active Controlled, Multicentre International Study to Compare the Response of Ultrasound-assessed Synovitis to Baricitinib, Alone and Combined with Methotrexate versus Etanercept in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-3-arm-randomized-open-label-parallel-active-controlled-multicentre-international-study-to-compare-the-response-of-ultrasound-assessed-synovitis-to-baricitinib-alone-and-combined-with-methotrexa/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-3-arm-randomized-open-label-parallel-active-controlled-multicentre-international-study-to-compare-the-response-of-ultrasound-assessed-synovitis-to-baricitinib-alone-and-combined-with-methotrexa/