Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) and hypereosinophilic syndrome (HES) are rare systemic diseases characterized by persistent eosinophilia and tissue infiltration, resulting in end-organ dysfunction (EOD)(1). Standard of care (SOC) includes corticosteroids, immunosuppressives, disease-modifying antirheumatic drugs and biologics(2). Mepolizumab, an anti-interleukin-5 monoclonal antibody, reduces disease manifestations and corticosteroid dependence(3,4). Real-world evidence on EOD outcomes following mepolizumab initiation in EGPA and HES is limited. This study evaluated mepolizumab impact on EOD compared with other SOC therapies in a US real-world setting.
Methods: This retrospective cohort study used administrative claims and EHR data from Optum’s Market Clarity database. Patients with EGPA (≥18 years) or HES (≥12 years) were identified at mepolizumab or SOC initiation (index). Patients were classified as mepolizumab users or chronic SOC users (≥6 months of >7.5 mg prednisone equivalent and/or ≥2 records of immunosuppressants or other disease-modifying antirheumatic drugs from index). Baseline sociodemographic characteristics, SOC use, comorbidities, and prior end-organ procedures were captured in the 6-months pre-index. Inverse probability of treatment weighting was used to balance the covariates between patient cohorts. EOD proportion, rate, and status were assessed ≥12 months post-index. Z-test compared weighted groups. Analyses were performed separately for EGPA and HES.
Results: Among 1102 patients with EGPA, 375 received mepolizumab and 727 received chronic SOC. Weighted analyses demonstrated numerical improvements in 14 distinct EOD conditions across multiple organ systems in those treated with mepolizumab vs chronic SOC. Significant improvements in EOD status were observed in acute kidney failure in mepolizumab vs chronic SOC (63.4% vs 23.4%, p=0.026) (Table).
Among 784 patients with HES, 198 received mepolizumab and 586 received chronic SOC. Weighted analyses showed numerical improvement across 12 distinct EOD conditions among those treated with mepolizumab vs chronic SOC. Significant improvements in EOD status were observed in mepolizumab vs chronic SOC in mitral regurgitation (90.9% vs 29.4%, p < 0.001), and venous thrombosis (69.5% vs 8.1%, p=0.009) (Table).
Conclusion: In this large real-world analysis, mepolizumab was associated with clinically meaningful improvements across multiple end-organ systems in both EGPA and HES, including cardiac, renal, and vascular conditions. These findings represent a potential signal of multisystem benefit and support further research to explore the durability and clinical significance of these effects.Funding: GSK (221044)References
Wechsler ME et al. J Allergy Clin Immunol. 2023;151(6):1415−28.
Khoury P et al. Mayo Clinic Proc. 2023;98(7):1054−70.
Wechsler ME et al. N Engl J Med. 2017;376(2):1921−32.
Roufosse F et al. J Allergy Clin Immunol. 2020;146(6):1397−405.
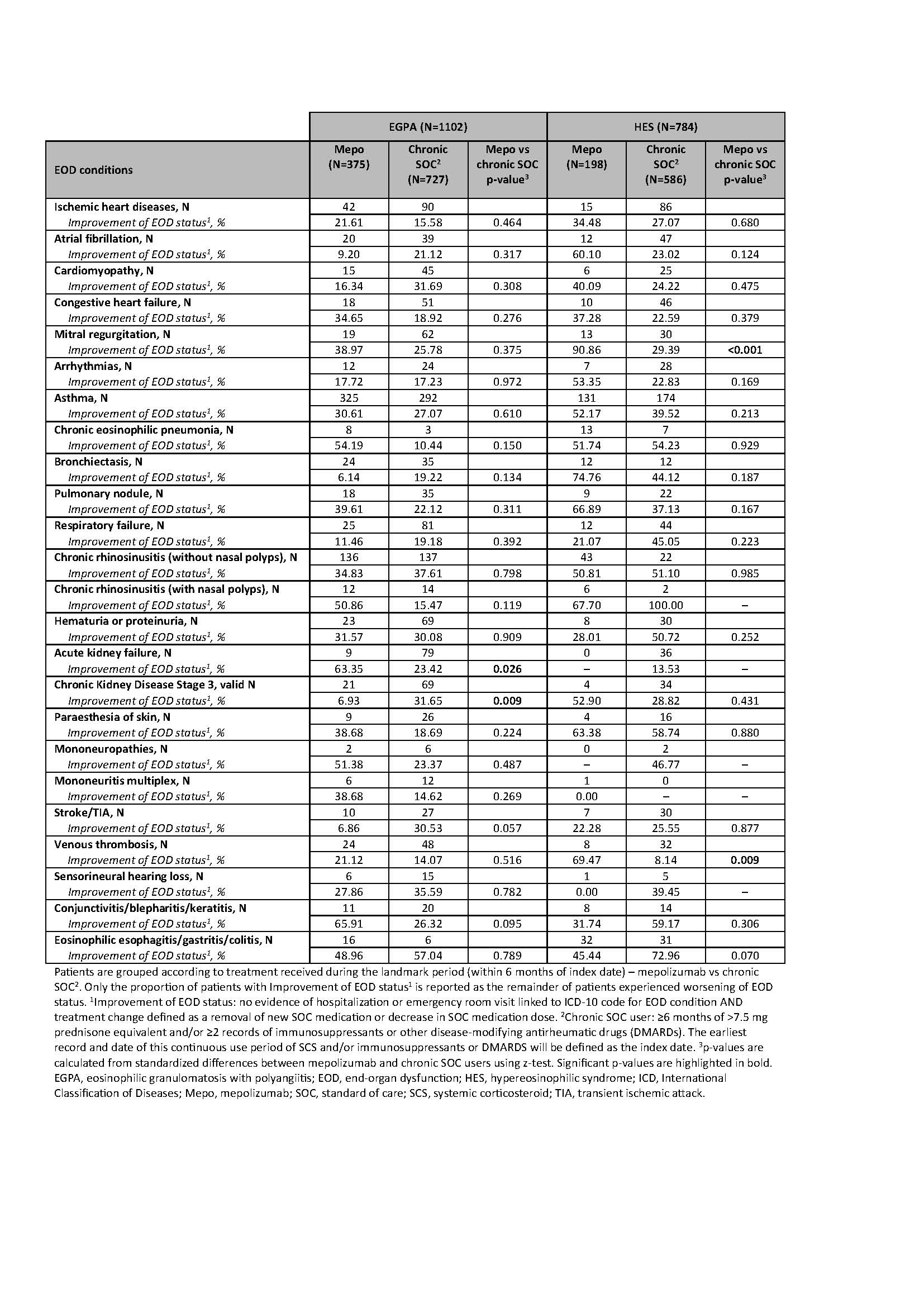 Table. Proportion of patients with EGPA and HES with EOD conditions with improvement of EOD status[1], stratified by treatment regimen (mepolizumab vs chronic SOC[2]).
Table. Proportion of patients with EGPA and HES with EOD conditions with improvement of EOD status[1], stratified by treatment regimen (mepolizumab vs chronic SOC[2]).
To cite this abstract in AMA style:
Silverman D, Barnes T, Odo N, Silver J, Le L, Edgecomb A, Patel H. Mepolizumab Reduces End-Organ Manifestations Compared with Standard of Care in Patients with EGPA and HES: A US Real-world Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/mepolizumab-reduces-end-organ-manifestations-compared-with-standard-of-care-in-patients-with-egpa-and-hes-a-us-real-world-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mepolizumab-reduces-end-organ-manifestations-compared-with-standard-of-care-in-patients-with-egpa-and-hes-a-us-real-world-analysis/
