Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: OA is a prevalent disabling disease growing globally due to aging populations and rising obesity. In primary OA, the effects of joint tissue damage accumulate with age, with low-grade inflammation and decline in the joint regenerative capacity.
A novel cell therapy of apoptotic PBMCs has been developed for OA based on robust pre-clinical data on their pro-resolution modulation of macrophage-mediated inflammation. Following a Phase I safety run-in, in which participants demonstrated favorable tolerability, we now report the pre-planned 3-month analysis results of a randomized controlled Phase IIa assessing safety and efficacy of apoptotic cell therapy in knee OA, as well as identifying a responder population based on baseline covariates.
Methods: ENX-CL-05-001 is a multicenter, randomized, double-blind, placebo-controlled Phase IIa in moderate–severe knee OA (NCT06233474). Participants received 3 knee injections on Days 0/14/28 and are followed to Month 12. Primary objectives were safety/tolerability; key secondary endpoints included changes in NRS and WOMAC pain scores and MRI semi-quantitative evaluations. Innovative machine-learning methods explored heterogeneity and high-responder populations.
Results: 134 patients were randomized and treated. Baseline characteristics were well balanced between the arms (mean age 61, 55% female, mean BMI 29). No treatment-related SAEs occurred. AEs were more frequent with apoptotic cell therapy vs placebo (89.6% [60/67] vs 71.6% [48/67]), driven by transient local reactions (typically resolving ~6 days vs ~10 days with placebo); noncompliance with treatments was uncommon (7.5% elected to forego subsequent injections due to AEs).
In the mITT population, pain NRS improvement was 24% higher with apoptotic cell therapy vs placebo; and WOMAC pain and function improvements were also numerically higher, however these did not reach statistical significance. An age-related responder population enriching for primary/idiopathic OA was identified (≥60 years; 69/129, 53.5% of study population) demonstrating significant improvements with apoptotic cell therapy: WOMAC Pain −27.82 (SD 20.76) vs −16.22 (SD 22.54), 72% relative improvement (p=0.03); WOMAC Total −26.43 vs −13.75, 92% relative improvement (p=0.012), including WOMAC Function −26.45 vs −12.63, 109% relative improvement (p=0.007). The 3-month pain NRS group difference (-0.98; 0-10), 48% relative improvement (p=0.07), was clinically meaningful and consistent with weekly pain reporting. Ongoing Month-6 readouts indicate potentially durable results. Moreover, pain improvement results were associated with more severe baseline imaging markers, including bone marrow lesions, Hoffa synovitis and radiographic structural damage.
Conclusion: Intra-articular apoptotic cell therapy was well tolerated without treatment-related SAEs and produced clinically meaningful and statistically significant improvements in pain and function endpoints in participants ≥60 years—consistent with an age-linked, macrophage-mediated OA phenotype. These findings support advancement into advanced trials program targeting older, primary/idiopathic knee OA.
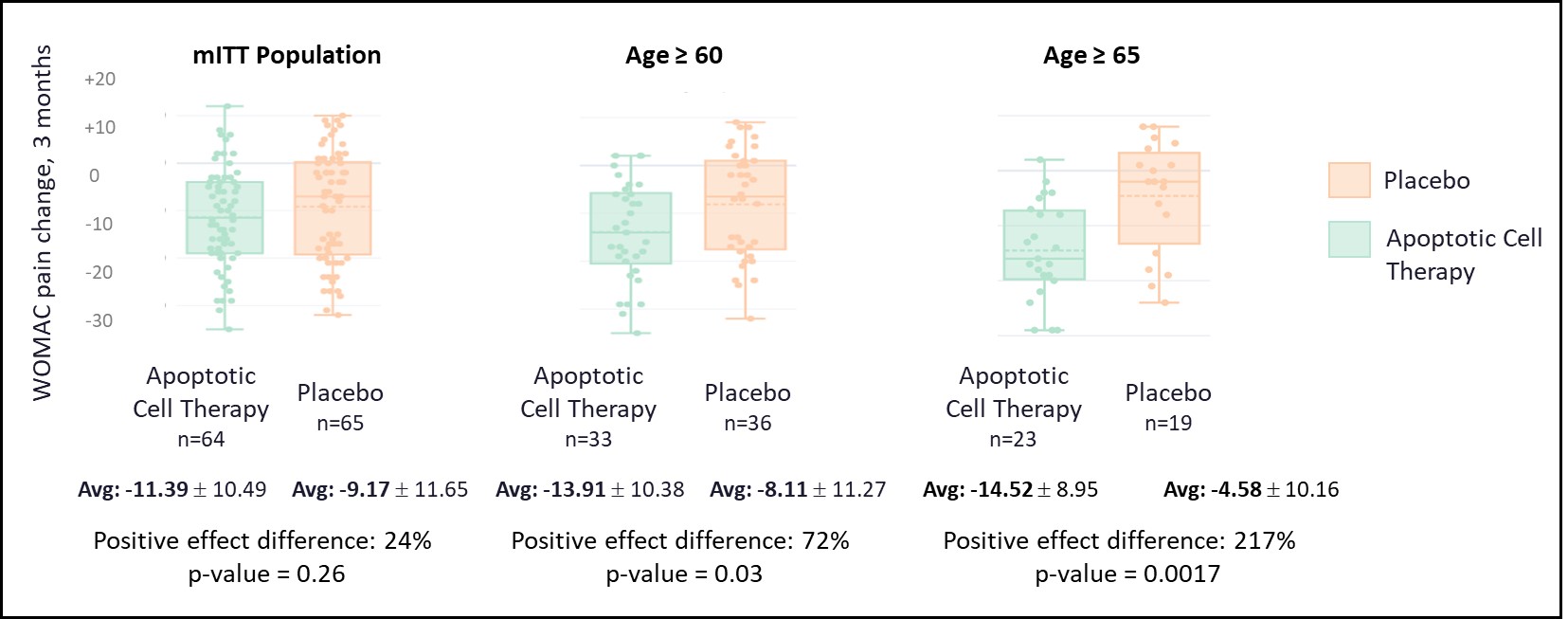 Figure 1: Results of WOMAC pain change at 3 months for the mITT overall population and Age-related Responder Population ≥ 60 years and ≥ 65 years
Figure 1: Results of WOMAC pain change at 3 months for the mITT overall population and Age-related Responder Population ≥ 60 years and ≥ 65 years
To cite this abstract in AMA style:
Conaghan P, Husøy B, Rovsing C, Boll S, Groppa L, Oron A, Bihlet A, Mobasheri A, Winkler T, Mevorach D, Galamidi E, Weinfeld-Bergman L, Binder L, Hershkovitz O. Randomized, Double-Blind, Placebo-Controlled Phase IIa Trial of an Innovative Intra-Articular Apoptotic Cell Therapy in Knee Osteoarthritis (OA): 3-Month Positive Outcomes and Identification of Responder Population (NCT06233474) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/randomized-double-blind-placebo-controlled-phase-iia-trial-of-an-innovative-intra-articular-apoptotic-cell-therapy-in-knee-osteoarthritis-oa-3-month-positive-outcomes-and-identification-of-r/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/randomized-double-blind-placebo-controlled-phase-iia-trial-of-an-innovative-intra-articular-apoptotic-cell-therapy-in-knee-osteoarthritis-oa-3-month-positive-outcomes-and-identification-of-r/
