Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s disease (SjD) is a chronic autoimmune disease whose pathogenesis is associated with aberrant activation of B-lymphocytes. Telitacicept, a novel fusion protein, dually targets and neutralizes B-lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL), and has previously demonstrated efficacy and safety in a phase 2 study in patients with SjD.
Methods: This randomized, double-blind, placebo-controlled, phase 3 study (NCT05673993) was conducted in 79 research centers in China. Participants aged 18–70 years meeting the 2016 ACR/EULAR criteria for SjD who were anti-SSA antibody positive with EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI)≥5 were eligible. Eligible participants were randomized (1:1:1) to telitacicept 160 mg, telitacicept 80 mg, or placebo groups to receive weekly subcutaneous injection for 48 weeks. During the period from Week 24–48, participants with inadequate response to treatment in the placebo group could switch to telitacicept 160 mg or telitacicept 80 mg at a ratio of 1:1 under blind conditions. The primary efficacy endpoint was the change from baseline in ESSDAI score at Week 24. Secondary endpoints included change from baseline in ESSDAI score at Week 48; proportion of participants with: ≥3-point reduction from baseline in ESSDAI score; ESSDAI score < 5; ≥1-point or ≥15% reduction from baseline in EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI); and change from baseline in ESSPRI total score (all at Week 24 and Week 48). The proportion of Sjögren's Tool for Assessing Response (STAR) responders was also assessed, along with adverse events (AEs) and immunological parameters.
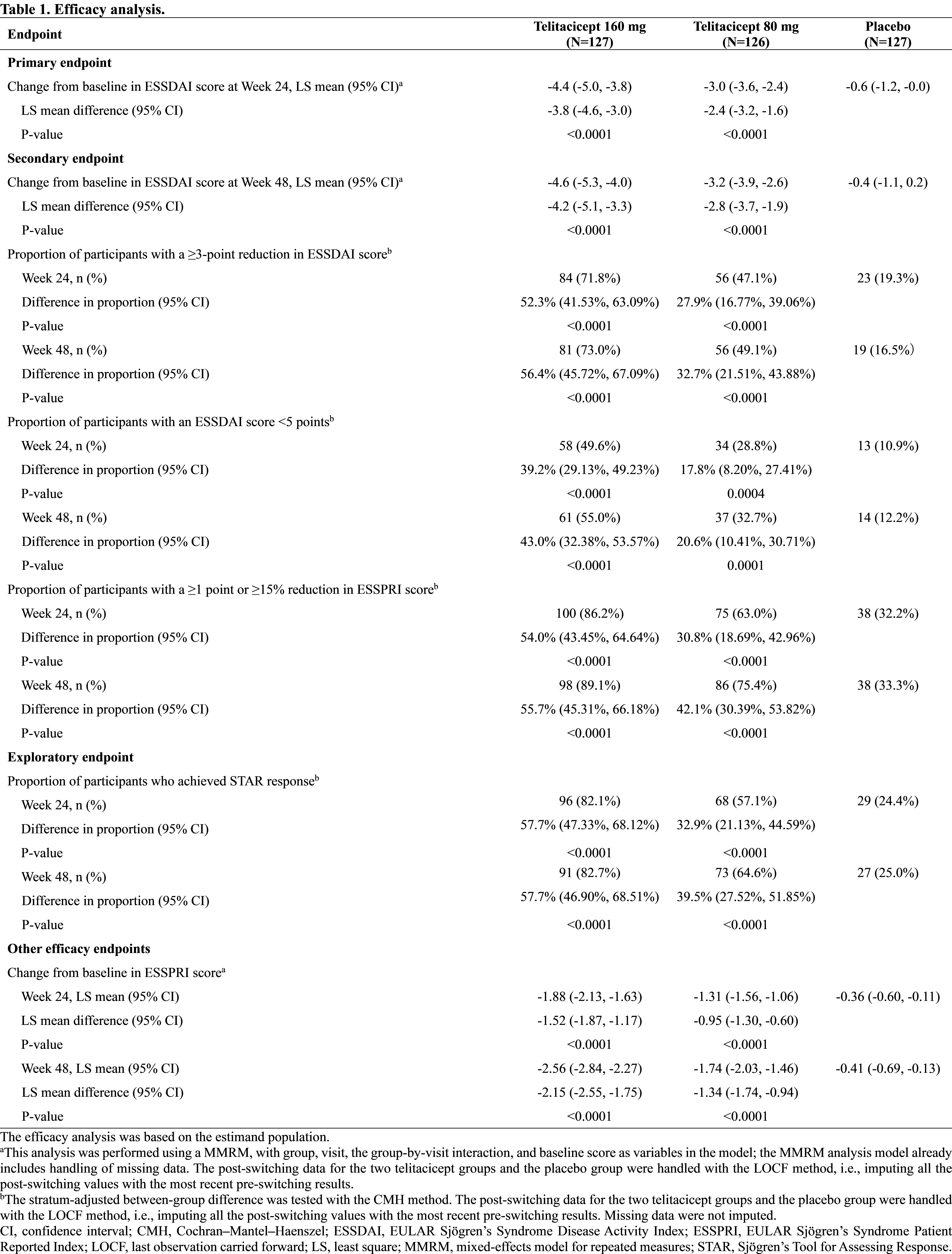
Results: A total of 381 eligible participants were randomly assigned to telitacicept 160 mg (Nf127), telitacicept 80 mg (Nf127), and placebo (Nf127). One participant in the telitacicept 80 mg group who prematurely withdrew before receiving study treatment was excluded from the estimand population and safety set. Compared with placebo, a statistically significant decrease in ESSDAI score from baseline at Week 24 was observed with telitacicept 160 mg (least square [LS] mean change: -4.4 vs -0.6, p < 0.0001) and telitacicept 80 mg (LS mean change: -3.0 vs -0.6, p < 0.0001) (Table 1). The efficacy in both telitacicept groups was maintained through Week 48 compared with placebo, with telitacicept 160 mg having a better reduction than 80 mg at Week 48 (LS mean change: -4.6 vs -3.2) (Figure 1a). Other efficacy endpoints, including proportion of participants who achieved ≥3-point reduction in ESSDAI score, proportion of participants with ESSDAI score < 5, proportion of participants who achieved ≥1-point or ≥15% reduction in ESSPRI score, change from baseline in ESSPRI score, and proportion of participants who achieved STAR response, showed consistent results with the primary endpoint (Table 1 and Figure 1). The majority of treatment-emergent AEs were mild or moderate (Table 2).
Conclusion: The primary efficacy endpoint was reached in this study. Compared with placebo, both telitacicept 160 mg and 80 mg demonstrated consistent clinical symptom improvement through 48 weeks and a favorable safety profile, with greater improvements observed with the telitacicept 160 mg dose.
To cite this abstract in AMA style:
Xu D, Zhang S, Qiao L, Zhang L, Wang W, Li L, Xiao B, Zhang J, Zuraw Q, Fang J, Zeng X. Efficacy and Safety of Telitacicept in Patients with Sjögren’s Disease: Results from a Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 3 Clinical Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-telitacicept-in-patients-with-sjogrens-disease-results-from-a-multicenter-randomized-double-blind-placebo-controlled-phase-3-clinical-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-telitacicept-in-patients-with-sjogrens-disease-results-from-a-multicenter-randomized-double-blind-placebo-controlled-phase-3-clinical-study/

.jpg)
.jpg)