Session Information
Session Type: Abstract Session
Session Time: 11:45AM-12:00PM
Background/Purpose: The recombinant vaccine against herpes zoster (HZ) (RZV) is recommended by ACR and EULAR for immunocompromised individuals. Short-term observational data from RA cohorts aged ≥50 years supports its overall reactogenicity and possible safety in non-randomized designs. However, the specific impact of different immunosuppressive drugs or combinations on vaccine immunogenicity is limited to small sample sizes or selected groups of patients, hampering conclusions about the effect of specific therapies. Moreover, new flare definition criteria using CDAI/SDAI were recently validated but the effect of vaccination using these new criteria were not evaluated. To address these gaps, we conducted a large prospective, randomized controlled trial to evaluate safety and assess humoral immunogenicity induced by RZV in RA adult (>18 years) patients.
Methods: RA patients were randomized to receive RZV (P1) or placebo (P2) at D0 and D42. Groups were blindly evaluated for disease activity (CDAI, SDAI and DAS28-CRP) at D0, D42 and D84. P2 group was vaccinated with a similar schedule at D84 and D126. Immunogenicity was evaluated in patients (P1+ P2) and compared to non-immunosuppressed control group (CG) at 1st RZV dose and 6 weeks post-vaccination. Anti-gE antibody concentrations (ELISA) were assessed at 1st RZV dose and six weeks after the second dose. Pre and post vaccination geometric mean titers (GMTs) and soroconversion (SC) were calculated. Adverse events (AE) were monitored for 3 months through scheduled visits and phone contacts.
Results: A total of 320 RA patients were randomized at D0: 165 in P1 and 155 in P2. The groups were balanced for age (p=0.562), sex (p=0.331), previous HZ infections (p=0.580), disease duration (p=0.838), rheumatoid factor (p=0.411) and anti-CCP (p=0.456) positivity, disease-modifying antirheumatic drugs (p >0.05), CDAI (p=0.268), SDAI (p= 0.343) and DAS28-CRP (p=0.268). After 2 vaccine doses, SC (96.6% vs. 100%, p=0.030) and GMT [8.3 (7.2-9.5) vs 12.9 (11.5-14.5 mUI/mL; p< 0.001] were significantly reduced in patients compared to CG. Linear regression corrected for pre-vaccination GMT linked older age (p=0.032), higher disease activity (p=0.042), MTX (p< 0.001), and prednisone use (p=0.045) with lower post-vaccination GMT. The concomitant use of MTX reduced post-vaccination GMT among bDMARD (p< 0.001) users (mainly TNFi users, p=0.003) and sDMARD users (p< 0.001). In multivariate analysis, higher pre-vaccination GMT persisted associated with higher post-vaccination GMT (p< 0.001), while older age (p=0.033) and MTX use (p< 0.001) remained associated with lower post-vaccination GMT. No HZ cases occurred by D84. Flare rates were comparable (p >0.05) between P1 and P2 (Figure 1) both at D42 and D84. No moderate/severe AEs were observed.
Conclusion: This large study showed that RZV was safe in immunosuppressed RA patients, with no evidence of increased flare rate using validated measures in comparison to placebo. Importantly, we identified MTX and older age as key factors that significantly impair humoral immune response. Although the humoral correlates of protection are not established, new strategies may be recommended for these patients (ClinicalTrials NCT05879419).
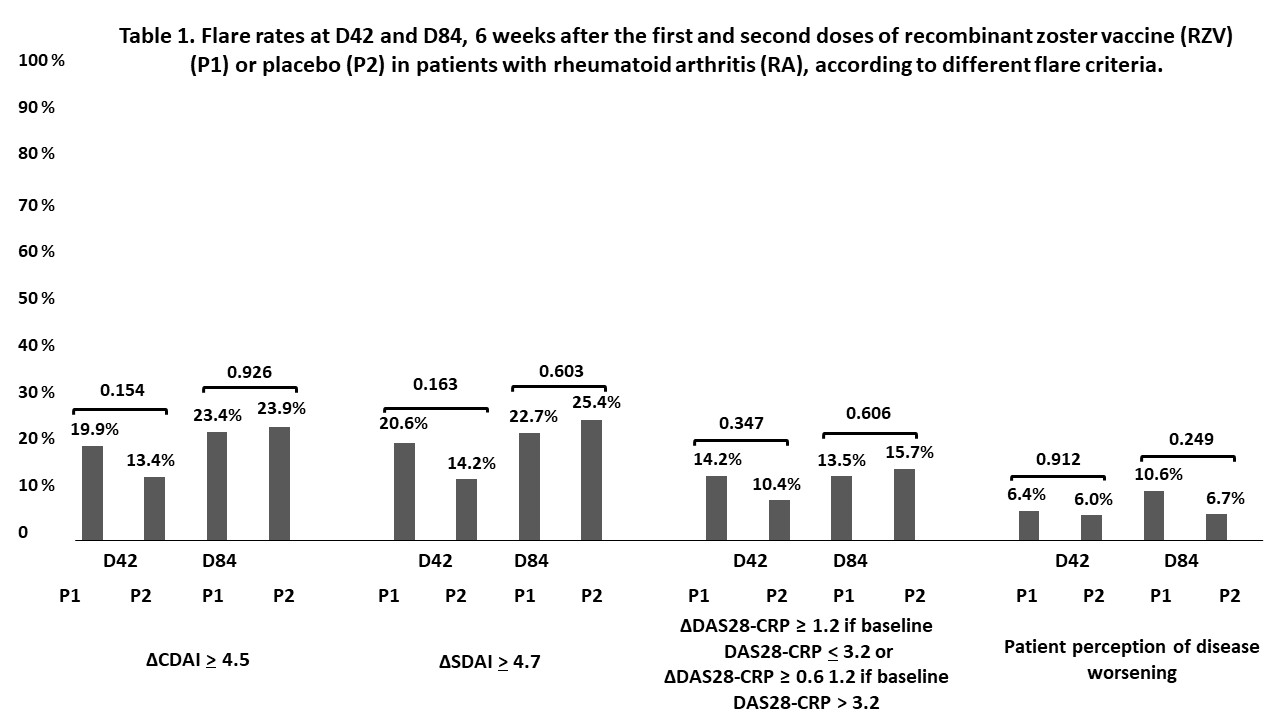 For safety analyses, all patients who adhered to protocol were included (n=275: 141 in P1 and 134 in P2). . Results are expressed in % and compared with the chi-square or Fisher’s exact test, as appropriate, as two-sided analyses; CDAI (Clinical Disease Activity Index), SDAI (Simplified Disease Activity Index), DAS28-CRP (Disease Activity Score using 28-joint count and C-reactive protein) and patient perception of disease worsening at D42 and D84 were compared to baseline status (D0). P1: pacients vaccinated with recombinant zoster vaccine (RZV) at D0 and D42; P2: patients who received placebo at DO and D42.
For safety analyses, all patients who adhered to protocol were included (n=275: 141 in P1 and 134 in P2). . Results are expressed in % and compared with the chi-square or Fisher’s exact test, as appropriate, as two-sided analyses; CDAI (Clinical Disease Activity Index), SDAI (Simplified Disease Activity Index), DAS28-CRP (Disease Activity Score using 28-joint count and C-reactive protein) and patient perception of disease worsening at D42 and D84 were compared to baseline status (D0). P1: pacients vaccinated with recombinant zoster vaccine (RZV) at D0 and D42; P2: patients who received placebo at DO and D42.
To cite this abstract in AMA style:
Medeiros-Ribeiro A, Farias L, Aikawa N, Pasoto S, Kupa L, Saad C, Shimabuco A, Bonfiglioli K, Domiciano D, Franco A, Silva C, Bonfa E. Methotrexate use and higher age impair humoral response against the recombinant herpes zoster vaccine (RZV) in Rheumatoid Arthritis: a prospective, randomized, placebo-controlled trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/methotrexate-use-and-higher-age-impair-humoral-response-against-the-recombinant-herpes-zoster-vaccine-rzv-in-rheumatoid-arthritis-a-prospective-randomized-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-use-and-higher-age-impair-humoral-response-against-the-recombinant-herpes-zoster-vaccine-rzv-in-rheumatoid-arthritis-a-prospective-randomized-placebo-controlled-trial/
