Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Pediatric Rheumatology – Clinical III (2675–2680)
Session Type: Abstract Session
Session Time: 12:00PM-12:15PM
Background/Purpose: This study assessed the timeline for the resolution of inflammation, changes in structural lesions at the sacroiliac joints (SIJ), and their correlation with patient-reported outcomes in youth with spondyloarthritis and axial disease (axJSpA) starting tumor necrosis factor inhibitor (TNFi) therapy.
Methods: This prospective, observational, multicenter study included youth aged 8 to 18 years with a clinical diagnosis of axJSpA starting TNFi therapy. TNFi therapy continued through the observation window as part of ongoing standard of care. Assessments at baseline and 12 weeks included a questionnaire, clinical exam, and MRI. Those with persistent SIJ inflammation at 12 weeks were invited for repeat MRI and questionnaires at 24 weeks. Imaging was independently assessed by 3 reviewers, blinded to timepoint and clinical details, using the Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ inflammation and structural scores (SIS and SSS). Patient-reported outcomes included PROMIS pain interference and global health scores, self-rated pain (neck/back/hip), and global change assessment (improved, stable, or worsened); Associations between imaging scores and patient-reported outcomes were analyzed using Spearman’s correlation.
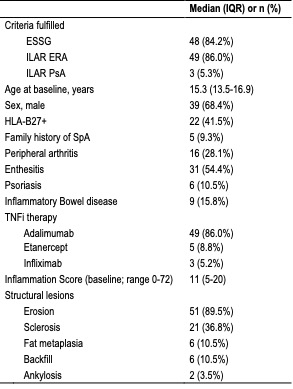
Results: Seventy-five participants enrolled; 73 (97.3%) completing the baseline visit. Among those, 62 (84.9%) had MRI findings consistent with axJSpA, and 57 of 62 (91.9%) completed the 12-week visit. Subject baseline characteristics are shown in Table 1. The median baseline SIS was 11 (IQR 5-20), and 89% of participants had ≥ 1 structural lesion, most commonly erosion. Change in imaging lesions from baseline to 12 and 24 weeks are illustrated in Figure 1. At 12 weeks, 89% improved in SIS (median change: -8 [IQR -18 to -3]); 63% achieved resolution of inflammation (SIS < 2). Of 26 with persistent inflammation and available global change data, 85% reported at least moderate improvement. At 24 weeks, 14 of 25 (25% of total) had ongoing inflammation with a median SIS of 1 (IQR 0-2). Erosion and sclerosis scores declined, increased or stayed the same in 58/25/17% and 21/9/70% of participants, respectively. Structural changes that reflect a tissue response to resolution of inflammation (fat metaplasia and backfill) demonstrated increased scores in 30% and 28%, respectively at week 12. Correlations between imaging outcomes and patient-reported measures at 12 weeks are shown in Figure 2.
Conclusion: Approximately half of participants had persistent SIJ inflammation on MRI at 12 weeks, yet 85% of those reported meaningful clinical improvement. Reductions in certain structural lesion scores were observed, and there was some evidence of early reparative changes, such as fat metaplasia or backfill. These findings underscore variability in therapeutic response to TNFi and indicate that significant clinical and imaging improvements may occur early in treatment, although a subset of patients may continue to exhibit active inflammation beyond 12 weeks. The modest correlations observed between imaging findings and patient-reported outcomes highlight the need for a multimodal approach to assessing treatment response in axJSpA.
.jpg) Figure 1. Lesion scores of each participant at baseline, 12 weeks, and 24 weeks for (A) Bone marrow edema (BME), (B) Erosions, (C) Sclerosis, (D) Fat metaplasia, and (E) Backfill. Each line represents a single participant and is color-coded to represent decreased (green), no change (black), or increased (red) in each lesion score from baseline to the final visit (12 or 24 weeks). Fat metaplasia and backfill are structural changes that reflect a tissue response to resolution of inflammation.
Figure 1. Lesion scores of each participant at baseline, 12 weeks, and 24 weeks for (A) Bone marrow edema (BME), (B) Erosions, (C) Sclerosis, (D) Fat metaplasia, and (E) Backfill. Each line represents a single participant and is color-coded to represent decreased (green), no change (black), or increased (red) in each lesion score from baseline to the final visit (12 or 24 weeks). Fat metaplasia and backfill are structural changes that reflect a tissue response to resolution of inflammation.
.jpg) Figure 2. Correlation matrix of patient-reported and imaging outcomes after 12 weeks of TNFi therapy. All raw scores generated from PROMIS instruments are translated into standardized T-scores with a population mean of 50 and standard deviation of 10. Higher scores in a domain represent more of the trait being measured; higher T-scores indicate a worse outcome in pain interference; lower T-scores indicate a worse outcome global health.
Figure 2. Correlation matrix of patient-reported and imaging outcomes after 12 weeks of TNFi therapy. All raw scores generated from PROMIS instruments are translated into standardized T-scores with a population mean of 50 and standard deviation of 10. Higher scores in a domain represent more of the trait being measured; higher T-scores indicate a worse outcome in pain interference; lower T-scores indicate a worse outcome global health.
To cite this abstract in AMA style:
Brandon T, Xiao R, Lovell D, Oberle E, Stoll M, Chauvin N, Francavilla M, Maksymowych W, Weiss P. Quantified Imaging Response at the Sacroiliac Joints to TNF-Inhibitor Therapy in Youth with Axial Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/quantified-imaging-response-at-the-sacroiliac-joints-to-tnf-inhibitor-therapy-in-youth-with-axial-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantified-imaging-response-at-the-sacroiliac-joints-to-tnf-inhibitor-therapy-in-youth-with-axial-disease/