Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical III (2651–2656)
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: Systemic sclerosis (SSc) heart involvement (SHI) is a serious disease manifestation associated with high mortality. This study presents newly developed classification criteria to enable standardised identification of SHI in clinical studies.
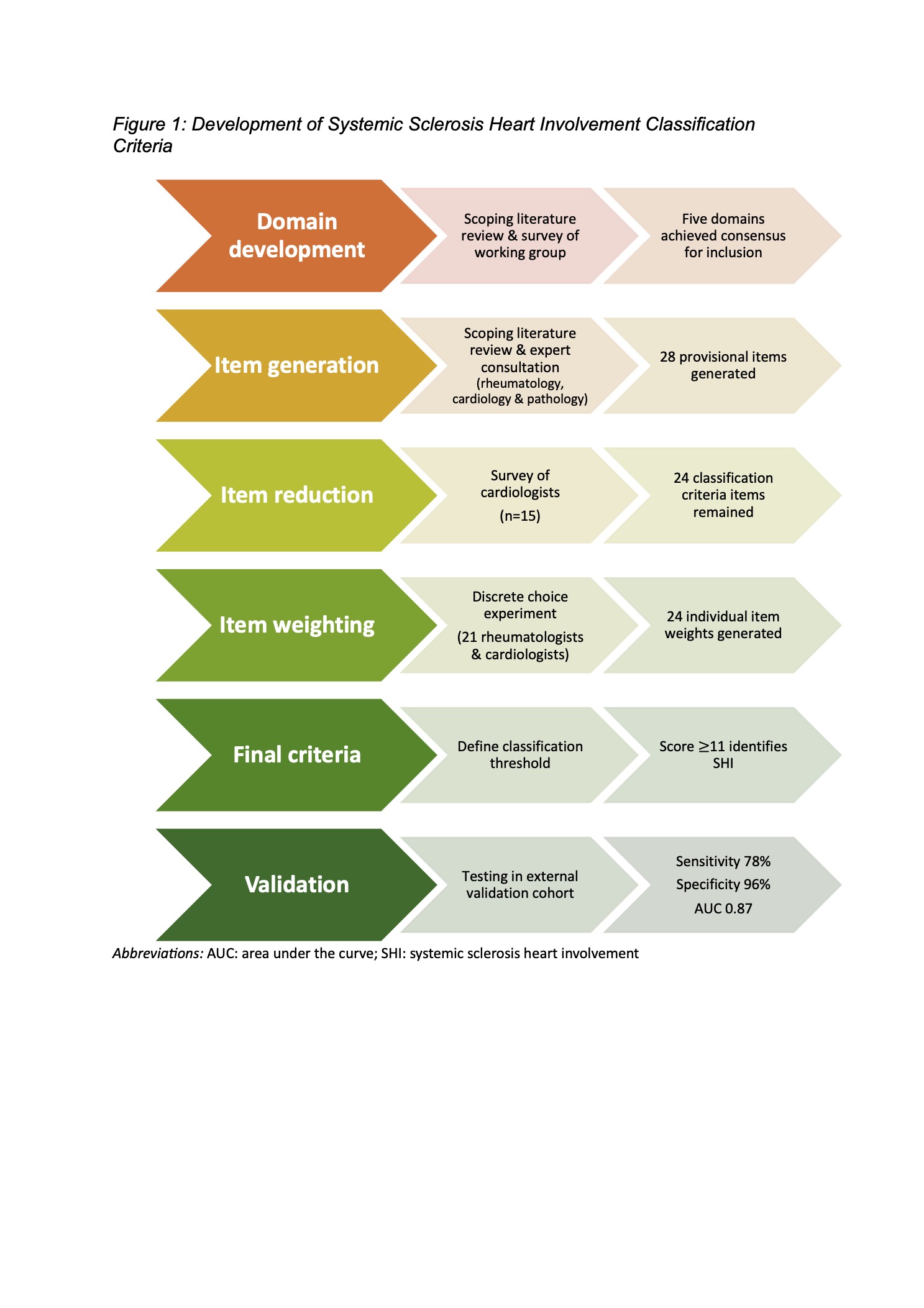
Methods: An international inter-disciplinary working group including rheumatology, cardiology and anatomical pathology members was assembled. Consensus methods were applied to determine the domains of cardiac disease to be included in the criteria and generate provisional items (Figure 1).Repeated consensus exercises and a discrete choice experiment were performed to reduce and weight items. A classification threshold was defined in a single centre derivation cohort (n=20), with emphasis placed on the specificity of the classification criteria. Performance of the criteria were tested against physician-diagnosed SHI, the current diagnostic gold standard.External validation studies were performed in a multi-centre validation cohort (n=168) to determine the sensitivity, specificity and discriminant validity of criteria by calculating the areas under receiver operator curves (AUC). Logistic regression analysis was performed to calculate the diagnostic odds ratio (OR) of the criteria classifying a patient as positive for SHI.
Results: The working group achieved consensus that criteria should be developed to identify the direct effects of SSc on the heart and discriminate SHI from cardiac complications of other disease manifestations, e.g. pulmonary arterial hypertension, renal disease. It was agreed that criteria should reflect the pathogenic changes of fibrosis, inflammation and vasculopathy known to affect the myocardium and pericardium in SSc.Five domains of cardiac disease reached the a priori threshold of 70% agreement for inclusion in the classification criteria. Twenty-eight provisional items were generated from the results of a literature review and expert consultation. Four items were omitted following item reduction survey, with the remaining 24 items weighted using the results of the discrete choice experiment (Table 1).A classification criteria score was calculated for each patient in the derivation cohort by summing individual criterion weights. A SHI classification score of ≥11 was chosen as it defined the the presence of SHI with a sensitivity of 100% and specificity of 82%. Application of this score to the validation cohort correctly classified 91% of cases, with a sensitivity of 78% and specificity of 96% and AUC of 0.87 (95%CI 0.80-0.93), compared to physician-diagnosed SHI (the current gold standard). A classification criteria score ≥11 gave an OR 82.60 (95%CI 26.47-257.72) for the presence of SHI.
Conclusion: These newly developed classification criteria are the first clinical criteria that can identify SHI with good sensitivity and excellent specificity. Their implementation in future studies will further validate their use and encourage systematic investigation of this important disease manifestation.
To cite this abstract in AMA style:
Ross L, Burns A, La Gerche A, Hansen D, Coghlan G, Stevens W, Prior D, Pham A, McKelvie P, Bellocchi C, Braun Moscovici Y, Bruni C, Carreira P, Frech T, Hoa S, Hudson M, Hsu V, Matucci-Cerinic M, Medina Fonseca B, Low A, Ng S, Rodriguez-Reyna T, Sahhar J, Talaat M, Proudman S, Vacca A, Baron M, Nikpour M. Development and Validation of the Scleroderma Clinical Trials Consortium Classification Criteria for Systemic Sclerosis Heart Involvement [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-the-scleroderma-clinical-trials-consortium-classification-criteria-for-systemic-sclerosis-heart-involvement/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-the-scleroderma-clinical-trials-consortium-classification-criteria-for-systemic-sclerosis-heart-involvement/

.jpg)