Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:15PM
Background/Purpose: To compare safety outcomes in patients with ankylosing spondylitis (AS) initiating Janus kinase inhibitors (JAKi) versus tumor necrosis factor inhibitors (TNFi).
Methods: This retrospective cohort study utilized data from the TriNetX Global Collaborative Network, comparing patients initiating JAKi (n=299) versus TNFi (n=299) after propensity score matching for age, sex, race, diabetes mellitus, hypertension, psoriasis, inflammatory bowel disease, uveitis, and hyperlipidemia, . Patients were followed for up to three years. Outcomes included mortality, major adverse cardiovascular events (MACE), venous thromboembolism (VTE), malignancy, gastrointestinal bleeding, hepatitis (non-infectious), serious infections (sepsis, pneumonia, herpes zoster), dermatologic events, and neuropsychiatric disorders. Kaplan–Meier analysis provided hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: JAKi initiation was associated with significantly increased all-cause mortality (4.3% vs. 3.3%; HR 4.94, 95% CI 1.59–15.37) and gastrointestinal bleeding (3.4% vs. 0%; p=0.002). Conversely, malignancy occurred exclusively among TNFi initiators (3.4% vs. 0%; p=0.001). Herpes zoster infection showed a non-significant trend toward higher incidence in JAKi users (6.0% vs. 5.0%; HR 1.90, 95% CI 0.95–3.83). No significant differences emerged in MACE, VTE, serious bacterial infections (including pneumonia and sepsis), dermatologic events, or hepatitis. Anxiety/insomnia diagnoses were numerically lower with JAKi (14.3% vs. 24.1%), but without statistical significance.
Conclusion: Compared with TNFi, JAKi therapy in AS was associated with increased mortality and gastrointestinal bleeding, but lower short-term malignancy incidence. Infection-related outcomes were similar, aside from a potential herpes zoster risk. These findings highlight distinct safety trade-offs, underscoring the need for individualized treatment choices in AS.
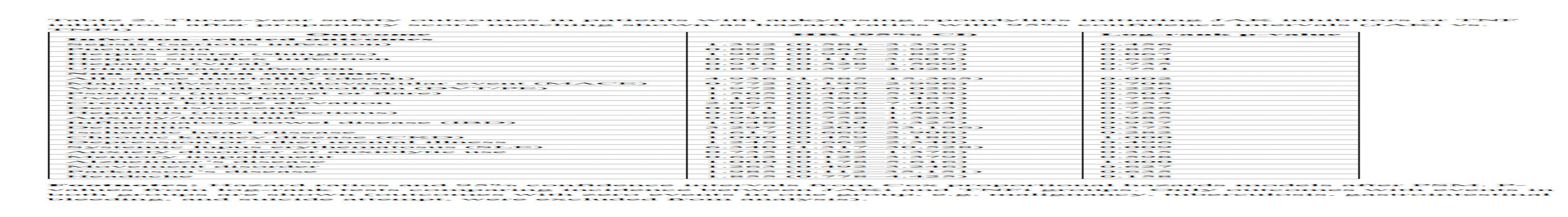 Table 1: Baseline Characteristics Before and After PSM (JAKi vs TNFi).
Table 1: Baseline Characteristics Before and After PSM (JAKi vs TNFi).
.jpg) Table 2. Three-year safety outcomes in patients with ankylosing spondylitis initiating JAK inhibitors or TNF inhibitors after propensity score matching shown as hazard ratios with 95% confidence intervals (JAKi vs. TNFi)
Table 2. Three-year safety outcomes in patients with ankylosing spondylitis initiating JAK inhibitors or TNF inhibitors after propensity score matching shown as hazard ratios with 95% confidence intervals (JAKi vs. TNFi)
To cite this abstract in AMA style:
Chen H. Comparative 3-year Safety Outcomes in Patients with Ankylosing Spondylitis Initiating JAK Inhibitor or TNF Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-3-year-safety-outcomes-in-patients-with-ankylosing-spondylitis-initiating-jak-inhibitor-or-tnf-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-3-year-safety-outcomes-in-patients-with-ankylosing-spondylitis-initiating-jak-inhibitor-or-tnf-inhibitor-therapy/
