Session Information
Date: Tuesday, October 28, 2025
Title: (2524–2546) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: IgG4-related disease (IgG4-RD) is a chronic immune-mediated disease typified by mass-forming lesions. Self-antigens driving the oligoclonal expansion of plasmablasts have previously been reported. However, broad screening of the IgG repertoire from IgG4-RD patients for reactivity across the human proteome has yet to be done. The goal of this study was to identify novel, potentially causal, disease-specific autoantigens by broadly screening the human proteome.
Methods: The HuProt microarray, displaying 21,216 proteins in total, including 15,889 unique human proteins, was used to screen plasma samples from IgG4-RD patients and controls across discovery and validation batches. The discovery batch included IgG4-RD (Nf30) and systemic sclerosis (SSc, Nf30). The validation batch included IgG4-RD (Nf42, 28 of which were not included in the initial batch), idiopathic pulmonary fibrosis (Nf20), sarcoidosis (Nf20), and healthy donors (Nf20). Microarray data was normalized and analyzed for autoantigens selectively bound by IgG4-RD samples after adjusting for false discovery rate. Autoantibodies with frequencies >15% among IgG4-RD were selected for unsupervised hierarchical clustering. A separate analysis focused on the highest titer autoantibodies across cohorts was also conducted. Selected autoantigens were validated using a luciferase immunoprecipitation system (LIPS) assay.
Results: FAM84A and LIMS1 were the two autoantigens that most accurately distinguished IgG4-RD from SSc in the discovery batch, while expected autoantigens distinguished SSc including those against centromeric proteins, RNA polymerase III subunits, and topoisomerase (Figure 1). Anti-FAM84A was present in 9/30 (30%) and anti-LIMS1 in 6/30 (20%) of the IgG4-RD cohort versus 0% in SSc and 1/30 (3%), respectively. The validation batch confirmed the presence of FAM84A and LIMS1 autoantibodies and identified four additional proteins to form a cassette of 6 autoantigens distinguishing IgG4-RD from HD. The additional four autoantigens included CCDC97, MAGEE1, ISM2 and SCG2. The nine anti-FAM84A samples from the discovery batch were reproducibly reactive to FAM84A in the validation batch. After excluding all overlapping samples between batches, frequencies of these autoantibodies were 7/28 (25%), 7/28 (25%), 14/28 (50%), 12/28 (43%), 7/28 (25%) and 5/28 (18%), respectively (Figure 2). Reactivity to at least one of these 6 autoantigens was observed in 35 of the 42 (83%) IgG4-RD patients and the IgG4-RD cohort additionally clustered into three distinct groups based on antigen specificity (Figure 3). Analysis focused on the highest titer autoantibodies identified ANXA11, as has been previously reported, and FAM84A. ANXA11 and FAM84A were validated by LIPS, with anti-FAM84A responses observed in 12% (23 of 192) of IgG4-RD patients and anti-ANXA11 in 12.5% (24 of 192). Anti-FAM84A patients showed enrichment for Mikulicz phenotype while anti-ANXA11 was linked to pancreatobiliary involvement.
Conclusion: The novel cassette of 6 autoantigens including FAM84A, LIMS1, CCDC97, MAGEE1, ISM2, and SCG2 distinguished IgG4-RD from controls. Further efforts to validate these findings are needed.
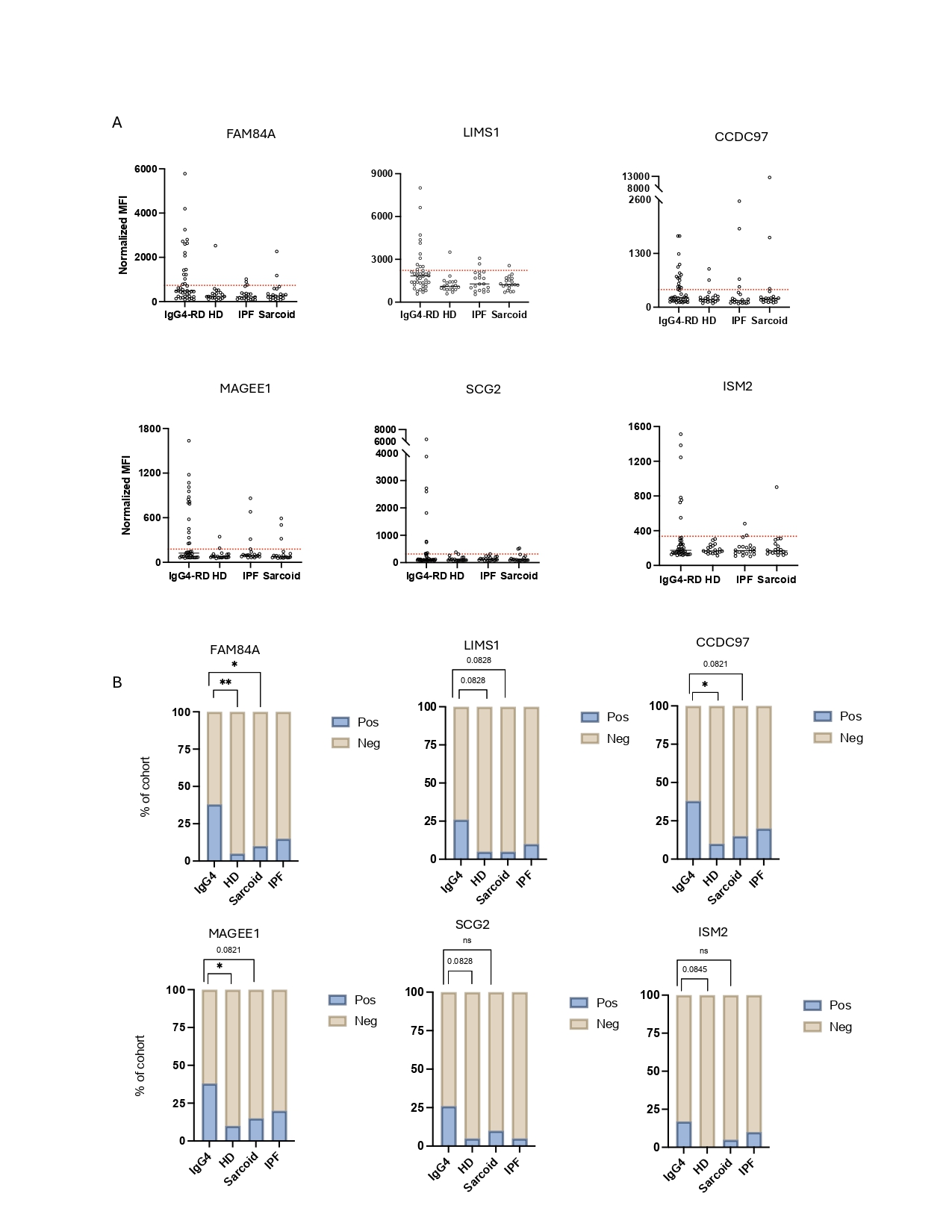 Figure 1. 51 differentially bound self antigens separate IgG4-RD from SSc patients by unsupervised hierarchical clustering. Heatmap with hierarchical clustering of differentially bound autoantigens between IgG4-RD (n=30) and SSc (n=29, 1 SSc patient was excluded.)
Figure 1. 51 differentially bound self antigens separate IgG4-RD from SSc patients by unsupervised hierarchical clustering. Heatmap with hierarchical clustering of differentially bound autoantigens between IgG4-RD (n=30) and SSc (n=29, 1 SSc patient was excluded.)
.jpg) Figure 2 Granularly selected cassette of 6 autoantigens distinguish IgG4-RD patients (n=42) from healthy donors (n=20), IPF (n=20) and Sarcoidosis (n=20). (A) Dot plots showing normalized mean flourescent intensity (MFI) values for each selected autoantibody, dotted red line represents cutoff value computed based on healthy donor cohort by the 1.5*interquartaile range (IQR) rule. (B) Bar charts representing % of subjects in each cohort with MFI values for each selected autoantigen that is above the cutoff described in Figure 1A. P value comparing frequencies between IgG4-RD with healthy donors and sarcoidosis patients was computed by Fisher’s exact test. Symbols: ns p value >0.05, *p ≤0.05, ** p≤ 0.01, *** p≤0.001, **** p≤0.0001.
Figure 2 Granularly selected cassette of 6 autoantigens distinguish IgG4-RD patients (n=42) from healthy donors (n=20), IPF (n=20) and Sarcoidosis (n=20). (A) Dot plots showing normalized mean flourescent intensity (MFI) values for each selected autoantibody, dotted red line represents cutoff value computed based on healthy donor cohort by the 1.5*interquartaile range (IQR) rule. (B) Bar charts representing % of subjects in each cohort with MFI values for each selected autoantigen that is above the cutoff described in Figure 1A. P value comparing frequencies between IgG4-RD with healthy donors and sarcoidosis patients was computed by Fisher’s exact test. Symbols: ns p value >0.05, *p ≤0.05, ** p≤ 0.01, *** p≤0.001, **** p≤0.0001.
.jpg) Figure 3. The casette of 6 novel autoantigens segregates the IgG4-RD cohort into 3 clusters and enrich for specific organd manifestations. (A) Heatmap with hierarchical clustering depicting the 6 selected autoantigens in the validation batch . Three main clusters are identified by the unsupervised clustering. (B) Dot plot showing relative light unit (RLU) values from LIPS assay validating FAM84A in a larger cohort IgG4-RD (n=192) compared to healthy donors (n=121) and SSc (n=56). Dashed line represents the cutoff based on mean + 3*standard deviations of the healthy donor cohort. P values were computed with Mann-Whitney U test. Among FAM84A positive IgG4-RD patients, 52% showed lacrimal gland involvement and 39% showed parotid gland involvement. Among ANXA11 positive IgG4-RD patients 70% showed pancreatic involvement.
Figure 3. The casette of 6 novel autoantigens segregates the IgG4-RD cohort into 3 clusters and enrich for specific organd manifestations. (A) Heatmap with hierarchical clustering depicting the 6 selected autoantigens in the validation batch . Three main clusters are identified by the unsupervised clustering. (B) Dot plot showing relative light unit (RLU) values from LIPS assay validating FAM84A in a larger cohort IgG4-RD (n=192) compared to healthy donors (n=121) and SSc (n=56). Dashed line represents the cutoff based on mean + 3*standard deviations of the healthy donor cohort. P values were computed with Mann-Whitney U test. Among FAM84A positive IgG4-RD patients, 52% showed lacrimal gland involvement and 39% showed parotid gland involvement. Among ANXA11 positive IgG4-RD patients 70% showed pancreatic involvement.
To cite this abstract in AMA style:
Bonaso F, Zhang Z, Guha M, Doyle I, Akaa J, McMahon G, Jha I, Montesi S, Guy T, Katz G, Wallace Z, Stone J, Mahajan V, Pillai S, Perugino C. Broad Screening of the Human Proteome Identifies a Cassette of Six Autoantigens that Distinguishes IgG4-Related Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/broad-screening-of-the-human-proteome-identifies-a-cassette-of-six-autoantigens-that-distinguishes-igg4-related-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/broad-screening-of-the-human-proteome-identifies-a-cassette-of-six-autoantigens-that-distinguishes-igg4-related-disease/
