Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Zetomipzomib (zeto), a selective immunoproteasome inhibitor, has previously shown anti-inflammatory activity in patients (pts) with SLE and LN in the open-label MISSION study. The PALIZADE trial (NCT05781750) was a global Phase 2b, multi-center, randomized (1:1:1), controlled double-blind study comparing the efficacy and safety of weekly subcutaneous administration of zeto 30 mg or 60 mg with placebo (pbo) in pts with active LN (Class III, IV ± V) on background standard of care therapy of MMF or equivalent plus corticosteroids. An Independent Data Monitoring Committee recommended pausing enrollment and dosing following 4 fatalities (1 – pbo, 2 – 30 mg arm, 1 – 60 mg arm) and additional nonfatal treatment-emergent serious adverse events (TESAE) that showed a common pattern of symptoms or proximity to dosing. PALIZADE was subsequently terminated early by the sponsor for strategic business reasons.
Methods: Planned enrollment was 279 pts, including 249 pts with Class III or IV ± V LN and 30 pts with pure Class V with a planned duration of 52 weeks of treatment and a primary endpoint at Week (W) 37. At the time of termination (October 2024), 84 patients (74 with Class III or IV ± V LN) were enrolled with mean treatment duration of 16.7 weeks. All enrolled pts were included in the safety analysis, and 39 pts with proliferative LN who reached W25 for 24-hour urine protein creatinine ratio (UPCR) evaluation were included in the efficacy analysis. No pt completed the full 52 weeks of treatment. Preliminary data on changes in proteinuria, median UPCR, SLEDAI-2K, and key lupus serologic markers at W25 are presented here. Whole blood transcriptomic analysis was performed.
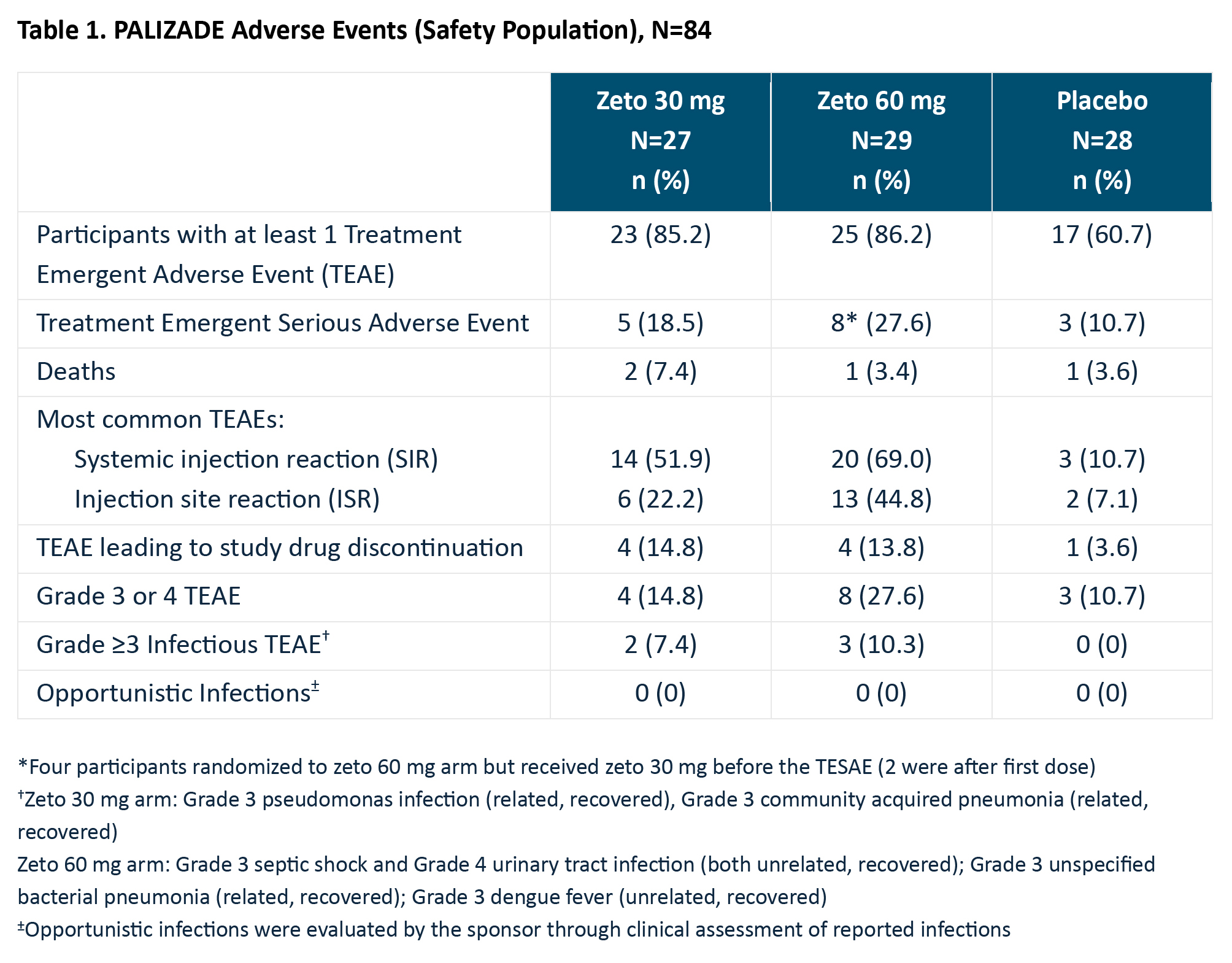
Results: Ninety-three percent of enrolled pts were female with mean age of 32.1 years, mean LN duration of 3.7 years, mean UPCR of 3.5 mg/mg, and a mean SLEDAI-2K score of 11.3. The most common TEAEs were injection site reaction (98% were Grades 1 or 2) and systemic injection reaction (97% were Grades 1 or 2), Table 1. Four fatalities (4.8%) and 16 total TESAEs (in 19% of pts) occurred. Evidence suggestive of co-morbidities immediately prior to study drug administration (e.g., possible systemic infection) and/or possible underlying disease characteristics (e.g., cardiovascular) were present in fatal events.Among the 39 evaluable pts with Class III/IV ± V LN, 42% (5/12) of those treated with 60 mg zeto achieved a UPCR ≤0.5 at W25. The median percent change in UPCR from baseline was -67% at W13 and -79% at W25, suggesting a rapid improvement in proteinuria with 60 mg zeto administration, Table 2. Additionally, improvements in SLEDAI-2K, serologic markers of SLE including anti-dsDNA, C3 and C4 were observed in both zeto arms.
Conclusion: Safety analysis of enrolled pts indicated overall similar safety profiles between zeto dosed at 30 mg and 60 mg, and these findings are consistent with previous studies of zeto in LN. Preliminary efficacy data from a treatment evaluable population with Class III/IV ± V LN in the PALIZADE trial offer encouraging activity in pts receiving a 60 mg dose of zeto.
To cite this abstract in AMA style:
Furie R, Anand N, Desai S, Lowe E, Muchamuel T, Palaniswamy K, Peterson R, Ray K, To Z, Whang J, Leff R. Efficacy and Safety Results of Zetomipzomib from the PALIZADE Phase 2b Clinical Trial in Patients with Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-results-of-zetomipzomib-from-the-palizade-phase-2b-clinical-trial-in-patients-with-lupus-nephritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-results-of-zetomipzomib-from-the-palizade-phase-2b-clinical-trial-in-patients-with-lupus-nephritis/

.jpg)