Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Antinuclear antibody (ANA) and SLE-related autoantibody testing are integral parts of SLE screening, diagnosis, and monitoring. However, these tests rely on access to phlebotomy for blood sample collection and a lab that can receive and perform these assays in a timely manner. This is a challenge for patients living in remote areas, including Indigenous populations and African countries, where SLE is likely underdiagnosed. Here, we investigated the performance of ANA and SLE-related autoantibody tests on dried blood spots (DBS) as a more resource-efficient and minimally invasive alternative for patients.
Methods: Patients with SLE meeting the 2012 SLICC or 2019 ACR/EULAR criteria or rheumatoid arthritis (RA) meeting the 2010 ACR/EULAR criteria as disease controls were enrolled from a single tertiary care centre. Serum was collected by both venipuncture and DBS at the same visit. DBS cards were subsequently stored under four conditions: 1) 25°C (room temperature) for one week, 2) 25°C for 4 weeks, 3) 4°C for one week, and 4) 4°C for 4 weeks. For each condition, ANA was performed using an indirect immunofluorescence assay (IFA) on HEp-2 substrate (NovaLite,Werfen, CA). ANA patterns were classified according to the most recently updated International Consensus on ANA Patterns recommendations. Per manufacturers’ directions, a positive test was defined as a titer of >=1:80. SLE-related antibodies (anti-U1-RNP, -histone, Jo-1, -ribosomal P, -Sm, -Sm-RNP, -Scl70, -PmScl, -centromere B, -PCNA, -Ro52/TRIM21, -Ro60/SSA, and -La/SSB) were performed on Connective-13 profile (addressable laser bead immunoassay by Theradiag, positive >80 AU/mL) and anti-dsDNA by ELISA (Werfen, CA, positive >300 IU/mL). We compared the ANA (titres and patterns) and autoantibody titres between venipuncture vs. DBS under the different storage conditions.
Results: 42 patients were enrolled (24 SLE, 18 RA). There was no statistical difference in the frequency of ANA positivity between venipuncture vs. DBS under all four storage conditions for SLE and RA controls (Fig1A). The ANA titres as indicated by intensity level measured using the automated digital IFA microscope (NovaView, Werfen) did not change significantly from week 1 to 4 at 25°C and 4°C. Most ANA patterns were the same between venipuncture and DBS after 1 week at both 25°C (79.5%) and 4°C (71.4%) (Fig2). There were more discrepancies in ANA pattern between venipuncture and DBS after 4 weeks for both 25°C (57.5%) and 4°C (52.5%). The correlation between venipuncture vs. DBS SLE-related autoantibodies was moderate to strong for most, except for anti-dsDNA, anti-histone, and anti-Scl70 antibodies (Table 1). Specifically, the correlation for anti-dsDNA was r=0.68 (p< 0.001).
Conclusion: In the study, we demonstrated that DBS performance is comparable to venipuncture for ANA and SLE-related autoantibody testing. Antibody titre did not vary significantly over time or with temperature variation. This highlights the sustainability of DBS as a potential screening test in under-resourced populations.
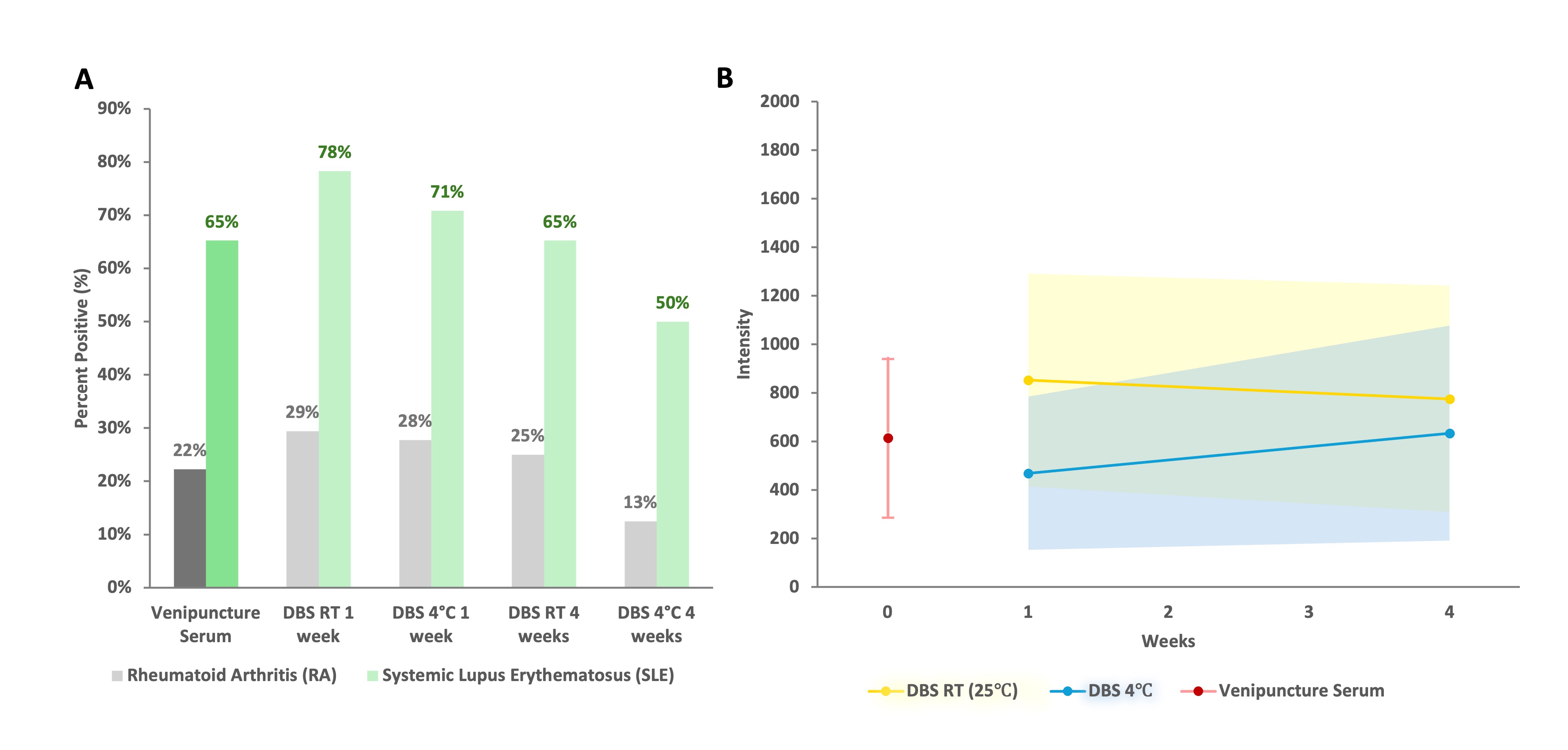 Figure 1. Venipuncture vs. DBS for A) ANA positivity (>=1:80) using indirect immunofluorescence on HEp2 substrate, stratified based on diagnosis (RA vs. SLE). There was no significant difference between venipuncture vs. DBS for all storage conditions; B) ANA titre over time (31 patients for 25°C, 30 patients for 4°C) represented by intensity level measured by an automated digital IFA microscope (NovaView, Werfen). Mean titres ± 95% confidence interval (CI) for 25°C were not significantly different after 1 week (853.68 ± 438.80) vs 4 weeks (776.00 ± 466.01) (McNemar paired chi-square test, p=0.554). Similarly, there were no significant differences for 4°C after 1 week (469.67 ± 315.6) vs. 4 weeks (634.27 ± 442.57) (p=0.321).
Figure 1. Venipuncture vs. DBS for A) ANA positivity (>=1:80) using indirect immunofluorescence on HEp2 substrate, stratified based on diagnosis (RA vs. SLE). There was no significant difference between venipuncture vs. DBS for all storage conditions; B) ANA titre over time (31 patients for 25°C, 30 patients for 4°C) represented by intensity level measured by an automated digital IFA microscope (NovaView, Werfen). Mean titres ± 95% confidence interval (CI) for 25°C were not significantly different after 1 week (853.68 ± 438.80) vs 4 weeks (776.00 ± 466.01) (McNemar paired chi-square test, p=0.554). Similarly, there were no significant differences for 4°C after 1 week (469.67 ± 315.6) vs. 4 weeks (634.27 ± 442.57) (p=0.321).
.jpg) Figure 2. Venipuncture vs. DBS for A) ANA pattern, examples of ANA images shown and B) comparing pattern agreement under 4 different conditions. There was highest agreement at week 1, regardless of temperature condition. RT=room temperature. Full pattern match refers to identical pattern(s) between venipuncture and DBS. Partial pattern match refers to when there are multiple ANA patterns on venipuncture and at least one of those patterns matches a DBS pattern, but not all. No match refers to no patterns agree on venipuncture vs. DBS.
Figure 2. Venipuncture vs. DBS for A) ANA pattern, examples of ANA images shown and B) comparing pattern agreement under 4 different conditions. There was highest agreement at week 1, regardless of temperature condition. RT=room temperature. Full pattern match refers to identical pattern(s) between venipuncture and DBS. Partial pattern match refers to when there are multiple ANA patterns on venipuncture and at least one of those patterns matches a DBS pattern, but not all. No match refers to no patterns agree on venipuncture vs. DBS.
.jpg) Table 1. SLE-related autoantibodies and correlation between venipuncture and DBS titres (1 week stored at room temperature shown only). All autoantibodies were analysed using Connective-13 profile (addressable laser bead immunoassay by Theradiag, positive >80 AU/mL) except for anti-dsDNA which was analyzed using ELISA (Werfen, CA, positive >300 IU/mL). Pearson correlation was used to evaluate the concordance of autoantibody titres.
Table 1. SLE-related autoantibodies and correlation between venipuncture and DBS titres (1 week stored at room temperature shown only). All autoantibodies were analysed using Connective-13 profile (addressable laser bead immunoassay by Theradiag, positive >80 AU/mL) except for anti-dsDNA which was analyzed using ELISA (Werfen, CA, positive >300 IU/mL). Pearson correlation was used to evaluate the concordance of autoantibody titres.
To cite this abstract in AMA style:
Li M, Sciore P, Fritzler M, Clarke A, Ocampo W, Buhler k, Seni J, Crowshoe L, Mosher D, Choi M. Dried Blood Spots for Remote ANA and Autoantibody Screening [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/dried-blood-spots-for-remote-ana-and-autoantibody-screening/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dried-blood-spots-for-remote-ana-and-autoantibody-screening/
