Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are often avoided when treating rheumatoid arthritis (RA) patients with decompensated heart failure (HF), based on increased rates of HF exacerbation in a trial of advanced HF patients without RA. In RA, recent data suggest that reducing inflammation, including with TNFi, is beneficial for HF risk reduction. We sought to leverage a unique cohort with data from an RA randomized clinical trial (TNFi vs triple therapy), linked to longitudinal electronic health records (EHR), enabling comparison of major adverse cardiovascular events (MACE) and HF events after trial completion.
Methods: We studied subjects from the Therapies for Active Rheumatoid Arthritis after Methotrexate (MTX) Failure (RACAT) trial, where 16 of the 36 sites were Veteran Affairs (VA) hospitals; participants were randomized to etanercept (ETA) plus MTX or triple therapy (hydroxychloroquine, sulfasalazine, MTX) for 48 weeks. Exclusion criteria included subjects with New York Heart Association class III or IV HF. Participants in the trial were linked to longitudinal VA EHR data, allowing assessment of outcomes after trial completion (4/2011). Participants were followed in the VA EHR from randomization (index date), for up to 5 years after trial completion. HF events and MACE related to atherosclerotic cardiovascular disease were defined by previously published ICD and CPT codes. As cross-over was allowed in RACAT at 24 weeks, the primary exposure was defined based on intention-to-treat (ITT) and as-treated (within the trial). We used unadjusted Cox regression to evaluate the association between the primary exposure (randomization to ETA+MTX versus triple therapy (referent)) and MACE, HF or death.
Results: Of 353 participants in RACAT, 66% were rheumatoid factor positive, and 44%* were followed at VA. Of these participants, 49% were randomized to ETA+MTX and 51% to triple therapy (Table 1). The mean age at randomization was 62 years, 89% were male, and 85% were self-reported White. Prior diagnoses of HF or MACE were present in 5% and 3% of the population, respectively, before randomization. After the 48-week trial period, TNFi use was more frequent in the group randomized to ETA+MTX (79%) compared to the triple therapy (43%). For ITT, incident MACE occurred in 8% of participants within 5 years of trial completion (Table 2), and the risk for incident MACE was comparable between the treatment groups (HR 1.25, 95% CI 0.42-3.72; Figure 1). Incident HF events occurred in 12% of participants within 5 years, and the risk for HF was comparable between the two treatment groups (HR 1.18, 95% CI 0.48-2.90). MACE, HF or death occurred in 25% of participants, and the risk was comparable between groups (HR 0.90, 95% CI 0.48-1.69). Similar effect estimates were observed in the as-treated analysis.* VA data privacy regulations require reporting as percentages due to some groups with n < 11
Conclusion: In this post-hoc analysis of a randomized controlled trial using EHR data to extend long term follow-up to 5 years after trial completion, we observed a similar incidence of MACE and HF irrespective of randomization to ETA+MTX or triple therapy.
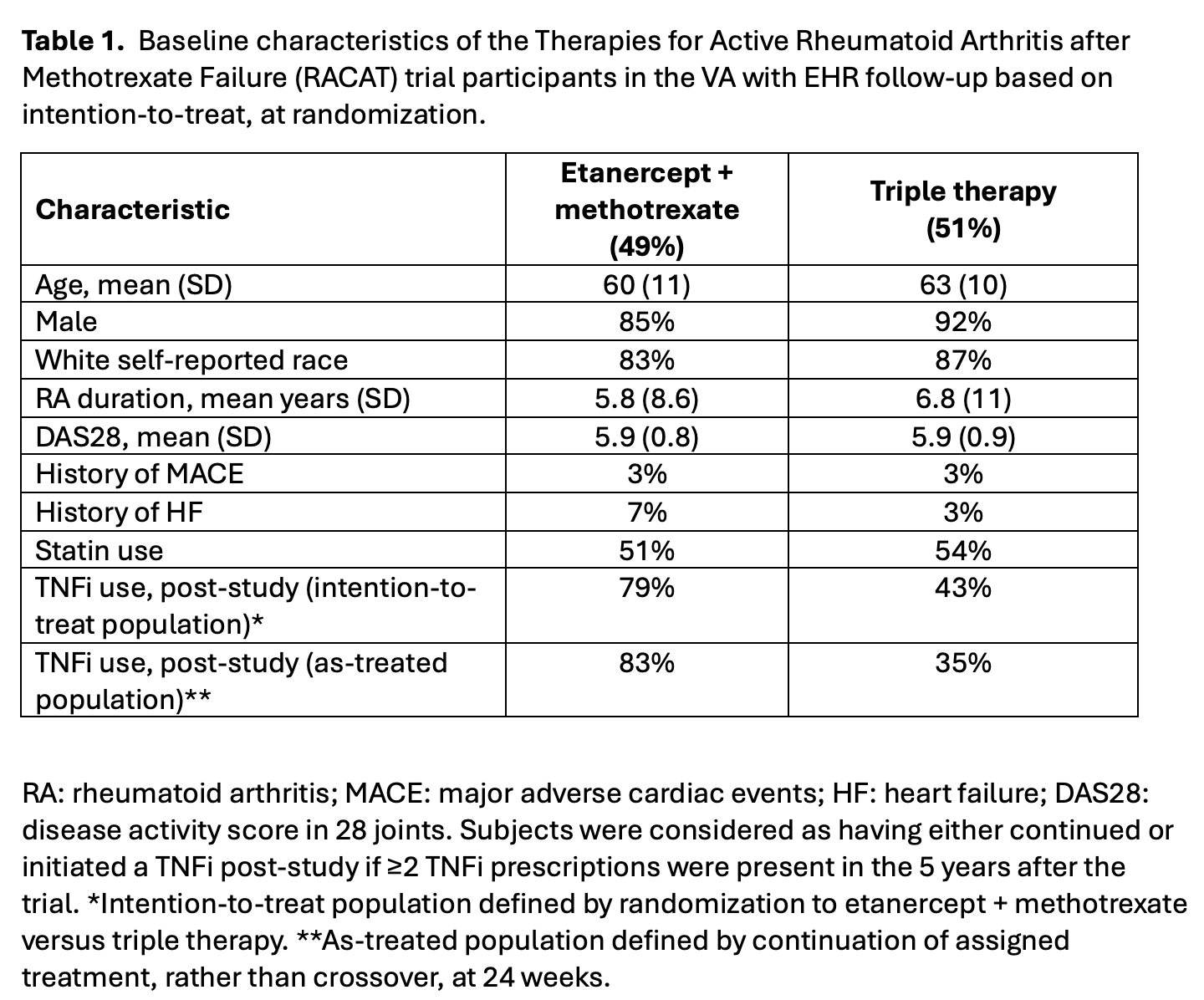 Table 1. Baseline characteristics of the Therapies for Active Rheumatoid Arthritis after Methotrexate Failure (RACAT) trial participants in the VA with EHR follow-up based on intention-to-treat, at randomization.
Table 1. Baseline characteristics of the Therapies for Active Rheumatoid Arthritis after Methotrexate Failure (RACAT) trial participants in the VA with EHR follow-up based on intention-to-treat, at randomization.
.jpg) Table 2. Incident MACE and HF events 5 years after trial completion in the intention-to-treat and as-treated populations.
Table 2. Incident MACE and HF events 5 years after trial completion in the intention-to-treat and as-treated populations.
.jpg) Figure 1. Hazard ratios for major adverse cardiac events and heart failure in patients randomized to etanercept plus methotrexate versus triple therapy in the intention-to-treat and as-treated populations.
Figure 1. Hazard ratios for major adverse cardiac events and heart failure in patients randomized to etanercept plus methotrexate versus triple therapy in the intention-to-treat and as-treated populations.
To cite this abstract in AMA style:
Hanberg J, Cheng D, Wang X, Sangar R, Ho Y, Costa L, Matty R, Feldman C, Johnson T, Baker J, England B, Gaziano J, Cho K, O'Dell J, Cannon G, Monach P, Mikuls T, Cai T, Liao K. Long-Term Cardiovascular Risk Following Tnfi vs Triple Therapy: A Post-hoc Analysis Integrating Randomized Clinical Trial and Electronic Health Record Data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-cardiovascular-risk-following-tnfi-vs-triple-therapy-a-post-hoc-analysis-integrating-randomized-clinical-trial-and-electronic-health-record-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-cardiovascular-risk-following-tnfi-vs-triple-therapy-a-post-hoc-analysis-integrating-randomized-clinical-trial-and-electronic-health-record-data/
