Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Intra-articular (IA) corticosteroid injections are commonly utilized for pain management in knee osteoarthritis (KOA). However, clinical characteristics associated with a greater benefit from corticosteroid above placebo are not well defined. We aimed to identify participant characteristics associated with superior response to corticosteroid plus lidocaine compared to lidocaine-only, leveraging data from a large multi-site crossover trial.
Methods: In this factorially-designed, double-blinded, randomized trial also evaluating an exercise incentive program, participants were randomized to IA corticosteroid injections with lidocaine or a lidocaine-only placebo, followed by crossover to the other injection at 16 weeks. Participants were age 40-80 years with KOA recruited from 4 VA Medical Centers, had a clinical indication for joint injection, and a Kellgren-Lawrence (K-L) radiographic grade of >1. The primary outcome was the change in the total Knee Osteoarthritis Outcome Score (KOOS) measured over multiple time points (bi-weekly) for 12 weeks after each injection (~1 month washout). The minimal clinically important difference (MCID) was defined as a change of >10. Participants that received both injections were included in the crossover trial analysis. For each participant, we determined the difference in the change in total KOOS score at each bi-weekly interval to determine the ‘corticosteroid-attributable benefit (CAB)’ (Figure 1). Linear regression incorporating generalized estimating equations identified participant characteristics associated with higher CAB 2-12 weeks after injection.
Results: Among 196 participants, several clinically relevant participant characteristics were associated with CAB (Table 1). For example, fibromyalgia (Nf11) was associated with lower CAB [B: -6.41 (95% CI: -12.55, -0.27), p=0.04] (Table 1). Participants in the lowest tertile of pain pressure threshold (PPT) (Nf63), suggesting greater pain sensitization, also had lower CAB (p=0.03) (Table 1); the lowest PPT tertile demonstrated smaller CAB compared to the highest (Figure 2). Never smokers (Nf103) (compared to current smokers) had higher CAB (p=0.02), and CAB was numerically greater among those with higher waist circumference per 1 cm (p=0.06) and among those with a KL grade of 2 (v. 1) (Nf42) (p=0.06), though these were not statistically significant (Figure 2). When excluding patients with fibromyalgia, KL-scores < 2, and current smokers, there was a significantly greater likelihood of achieving MCID when receiving corticosteroid compared to placebo [OR 2.02 (95% CI: 1.20, 3.40) p=0.008; NNT=10], while no benefit was observed in the overall sample [OR 1.36 (0.93, 1.98) p=0.11; NNT= 23].
Conclusion: In this analysis from a large crossover trial, KOA participants with fibromyalgia and greater pain sensitization experienced significantly less added benefit from IA corticosteroid compared to lidocaine only. These findings suggest reduced benefits of corticosteroids among those with evidence of pain sensitization and may help clinicians select patients most likely to benefit from IA corticosteroids for KOA.
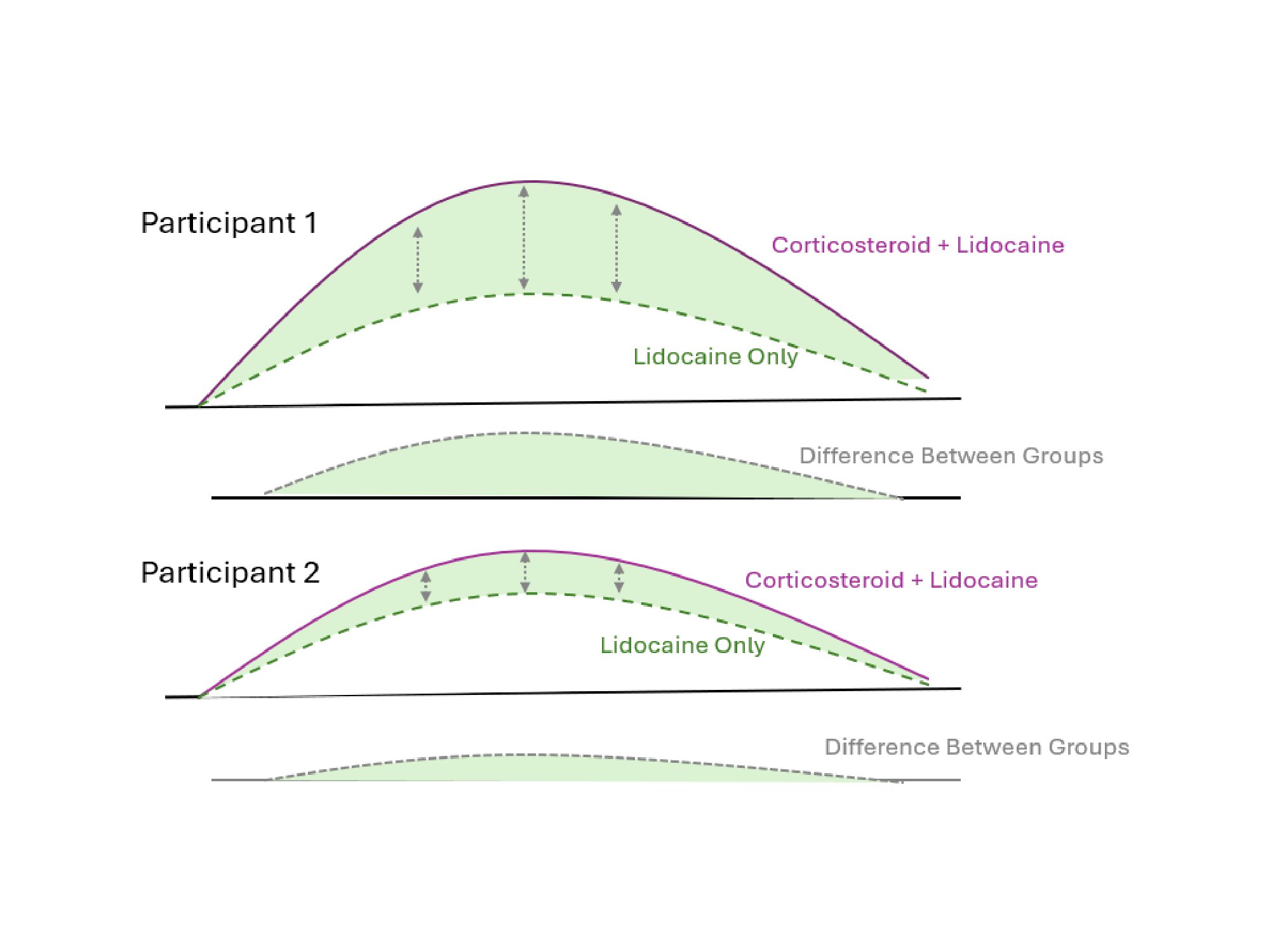 Table 1. Unadjusted associations between baseline characteristics and greater corticosteroid-attributable benefit from injections in the crossover trial analysis.
Table 1. Unadjusted associations between baseline characteristics and greater corticosteroid-attributable benefit from injections in the crossover trial analysis.
.jpg) Figure 1. Visualization of “Corticosteroid-Attributable Benefit” (CAB) as Difference in Change in Total KOOS Score at Each Weekly Interval. The CAB was calculated as the average difference in change in total KOOS score at each weekly interval.
Figure 1. Visualization of “Corticosteroid-Attributable Benefit” (CAB) as Difference in Change in Total KOOS Score at Each Weekly Interval. The CAB was calculated as the average difference in change in total KOOS score at each weekly interval.
.jpg) Figure 2. Corticosteroid-Attributable Benefit (CAB) for Each Tertile of Knee Pain Pressure Threshold (PPT). The lowest tertile of PPT demonstrated significantly lower CAB than the highest tertile (p=0.03).
Figure 2. Corticosteroid-Attributable Benefit (CAB) for Each Tertile of Knee Pain Pressure Threshold (PPT). The lowest tertile of PPT demonstrated significantly lower CAB than the highest tertile (p=0.03).
To cite this abstract in AMA style:
Keller N, England B, Wysham K, Quinones M, Olave M, Wetzel S, Brubeck H, Gillcrist R, Lavery C, Ateh B, Kramer B, Hayes K, Xiao R, Jin K, Ogdie A, White D, Neogi T, Scanzello C, Baker J. Responder Phenotype Analysis for Intra-Articular Injections: Secondary Analysis from a Large Multi-Site Crossover Clinical Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/responder-phenotype-analysis-for-intra-articular-injections-secondary-analysis-from-a-large-multi-site-crossover-clinical-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/responder-phenotype-analysis-for-intra-articular-injections-secondary-analysis-from-a-large-multi-site-crossover-clinical-trial/
