Session Information
Date: Tuesday, October 28, 2025
Title: (2052–2078) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Total Improvement Score (TIS), as defined by the ACR/EULAR myositis response criteria, is increasingly employed as a primary endpoint in clinical trials for dermatomyositis (DM). This study aims to assess the construct validity of TIS in a prospective, longitudinal cohort of patients with DM by examining the relationship between TIS improvement categories and Patient-Centered Outcome Measures (PCOMs) and Core Set Measures (CSMs) that assess various domains including fatigue, pain, quality of life, physical activity and physical function.
Methods: Participants met the 2017 EULAR/ACR criteria for probable or definite DM and were enrolled at the University of Pittsburgh Myositis Center between 2016 and 2018. Data collected at baseline, 3 months, and 6 months included manual muscle testing (MMT), physician and patient global activity (MDGA, PtGA), extra-muscular global activity (EMGA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and muscle enzyme levels. PCOMs included Short Form-36, PROMIS-Physical Function, sit-to-stand (STS), Timed Up-and-Go (TUG), 6-minute walk, pain and fatigue visual analogue scale (VAS), ActiGraph-based physical activity, and hand-held dynamometry. The TIS was calculated according to ACR/EULAR myositis response criteria, with TIS < 20 indicating no improvement and ≥20 indicating at least minimal improvement. Spearman correlation assessed relationships between TIS and outcome changes. Differences between the CSMs and PCOMs amongst patients with and without minimal improvements (TIS ≥20) were evaluated.
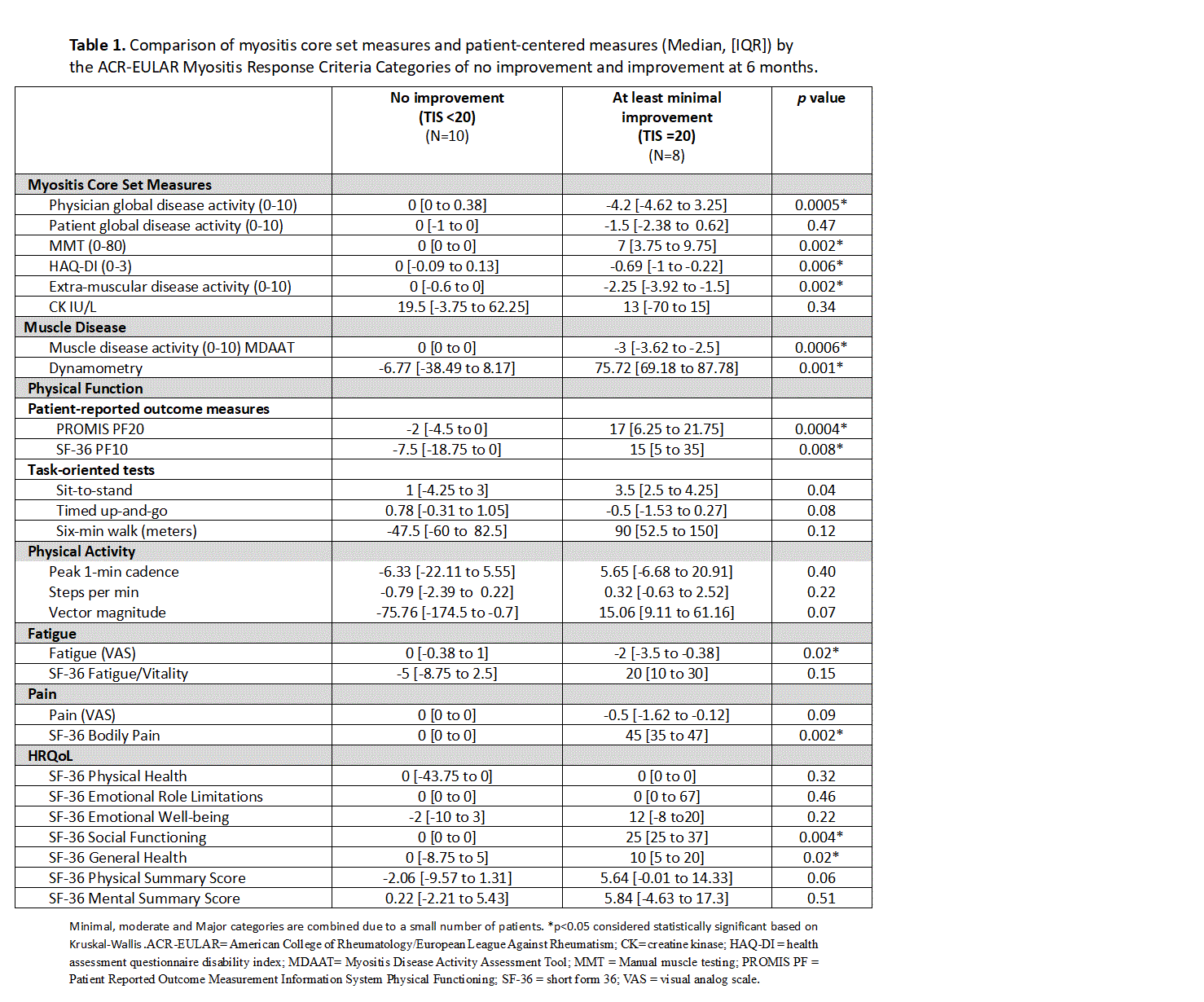
Results: Among n=24 participants, the mean age was 47 years; 71% were female and 96% were white. Patient-Reported Outcome Measures (PCOMs), including fatigue, pain, quality of life, muscle strength, disability index, and physical function, demonstrated significantly greater improvement in patients who met minimal improvement criteria by the TIS, compared to those with no improvement. Additionally, most CSMs, including MDGA, EMGA, and MMT, showed significant changes among those with minimal improvement compared to no improvement (Table 1). Among patients with active DM (defined as having active muscle activity at baseline; n=14) , those exhibiting at least minimal improvement on the TIS (≥20) at 6 months demonstrated average improvements across nearly all PCOMs. Correlations between TIS and changes from baseline in PCOMs and CSMs at 6 months revealed that improvement on most measures was associated with TIS. Strong correlations (rho > 0.5) with TIS were observed for physical function measures, handheld dynamometry, Timed Up-and -Go, and some quality of life measures.
Conclusion: The results support the construct validity of TIS and its use as a clinically meaningful endpoint in DM, capturing benefits in disease activity and aspects of pain, fatigue, function, quality of life, and overall well-being.
Table 1. Comparison of myositis core set measures and patient-centered measures (Median, [IQR]) by the ACR-EULAR Myositis Response Criteria Categories of no improvement and improvement at 6 months.
To cite this abstract in AMA style:
Chandrasekhara Pillai A, Keret S, Moghadam-Kia S, V. Oddis C, Aggarwal R. Construct Validity of Total Improvement Score (TIS) as an Endpoint for Clinically Meaningful Improvement in Multiple Patient-Centered Outcome Measures in Dermatomyositis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/construct-validity-of-total-improvement-score-tis-as-an-endpoint-for-clinically-meaningful-improvement-in-multiple-patient-centered-outcome-measures-in-dermatomyositis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/construct-validity-of-total-improvement-score-tis-as-an-endpoint-for-clinically-meaningful-improvement-in-multiple-patient-centered-outcome-measures-in-dermatomyositis/