Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Primary cardiac involvement is a common complication of Systemic sclerosis (SSc), characterized by fibrosis and diastolic dysfunction, presumably due to microvascular dysfunction and repeated ischemia reperfusion injury. Downregulation of transcription factor Fli1 in SSc contributes to vasculopathy and skin fibrosis, but its potential role in cardiac disease is unclear. Using mice with heterozygous deletion of Fli1 (Fli1+/-), we aim to uncover the effects of Fli1 downregulation on cardiac dysfunction in SSc through cardiac phenotypic studies and single-cell analyses.
Methods: Cardiac function was evaluated at 10-12 weeks in wild type and Fli1+/- mice using echocardiography. Sirius red staining followed by Image J analysis was used to assess cardiac collagen content. Cardiac apex tissue underwent mechanical and enzymatic dissociation for single-nuclei isolation using the 10X Genomics Nuclei Isolation Kit. The nuclei were barcoded using the 10X Chromium Single-Cell Platform, followed by cDNA library preparation. FASTQ files were generated and analyzed using BioTuring Single-Cell Analysis Software to identify distinct cellular clusters and transcriptional changes.
Results: Fli1+/- mice exhibited diastolic dysfunction, with elevated E/e’ and prolonged isovolumic relaxation time, as well as reduced systolic function indicated by decreased ejection fraction and fractional shortening (Fig. 1A). Sirius red staining confirmed increased collagen deposition in Fli1+/- hearts (Fig. 1B). Single-cell transcriptomic analysis identified 13 major cell types (Figure 2A). Cardiomyocyte cluster analysis revealed a transcriptional shift, with appearance of a novel, mutant specific cluster, and loss of a wild type cluster (Figure 2B). Differential gene expression analysis between these two clusters revealed several upregulated genes including PDK4 (log2FC=3.3 FDR=1.36e-23), and ANGPTL (log2FC=2.18 FDR=7.54 e-64). Analysis of the enriched pathways revealed upregulation of β-fatty acid oxidation in Fli1+/- cardiomyocytes (2.78e-15), a metabolic shift previously linked to impaired cardiomyocyte proliferation following ischemia-reperfusion injury. Additionally, macrophages from Fli1+/- mice showed increased expression of PLA2G7, a gene linked to inflammatory remodeling and fatty acid metabolism in cardiac macrophages. Supporting a role in cardiac fibrosis, Fli1+/- fibroblasts displayed elevated expression of fibrosis related genes including LOX, COL20A1, TGFBR3L, CCN2, SERPINE1, LOXL2, NOX4, and IL6RA.
Conclusion: Fli1 haploinsufficiency drives cardiac dysfunction and fibrosis via metabolic reprogramming in cardiomyocytes and macrophages with enhanced fatty acid oxidation and profibrotic activation of fibroblasts through TGF-β/LOX pathways. This suggest that Fli1 deficiency may be a contributor to primary cardiac involvement in SSc.
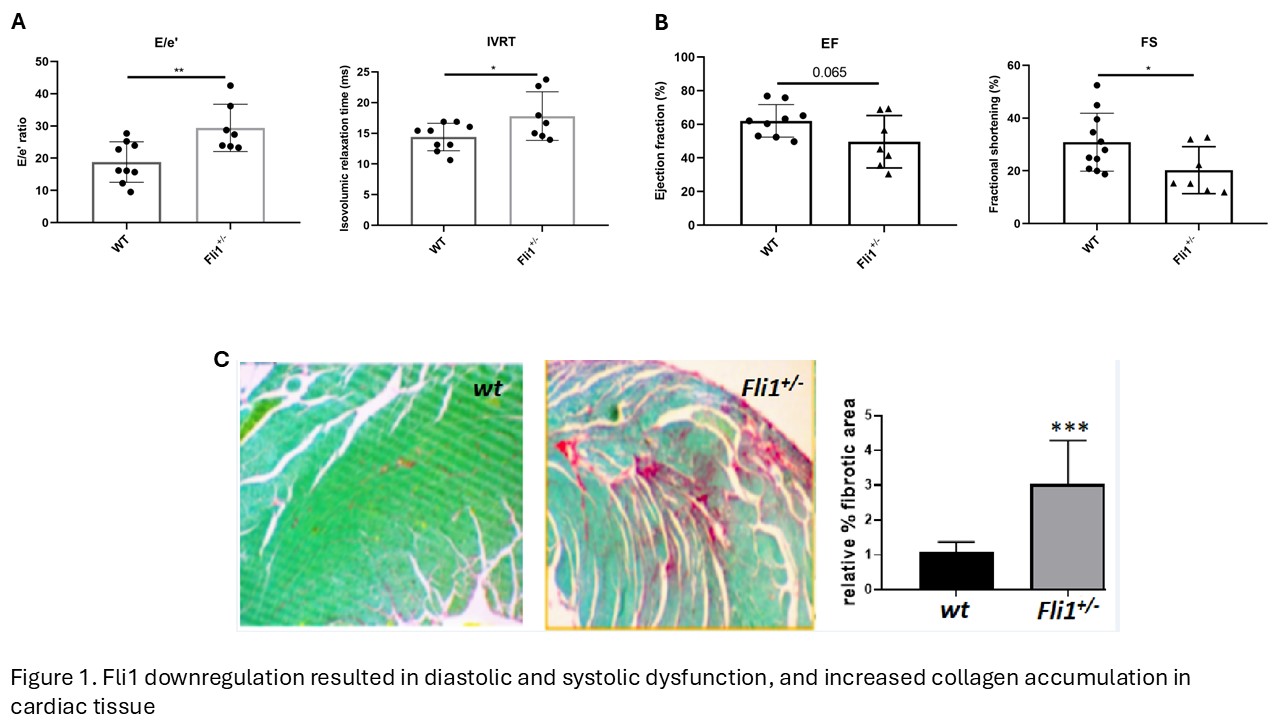 Fli1 downregulation resulted in diastolic and systolic dysfunction, and increased collagen accumulation in cardiac tissue
Fli1 downregulation resulted in diastolic and systolic dysfunction, and increased collagen accumulation in cardiac tissue
.jpg) UMAP of cardiac tissue cells, upregulated pathways and differentially expressed genes in cardiomyocytes, fibroblasts and macrophages
UMAP of cardiac tissue cells, upregulated pathways and differentially expressed genes in cardiomyocytes, fibroblasts and macrophages
To cite this abstract in AMA style:
Moyo K, El Adili F, Trojanowska M, Bujor A. Global Downregulation of Fli1 in Mice Induces Cardiac Dysfunction via Enhanced β-fatty acid Oxidation and Collagen Deposition [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/global-downregulation-of-fli1-in-mice-induces-cardiac-dysfunction-via-enhanced-%ce%b2-fatty-acid-oxidation-and-collagen-deposition/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/global-downregulation-of-fli1-in-mice-induces-cardiac-dysfunction-via-enhanced-%ce%b2-fatty-acid-oxidation-and-collagen-deposition/
