Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Immunological Complications of Medical Therapy (1728–1733)
Session Type: Abstract Session
Session Time: 11:00AM-11:15AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have become essential in cancer treatment, offering significant survival benefits across various malignancies. However, ICIs can cause immune-related Adverse Events (irAEs) and potentially exacerbate pre-existing autoimmune diseases. Rheumatologic diseases (RDs) are frequently excluded from clinical trials of ICIs due to these concerns. Understanding flare incidence is crucial for optimizing cancer management and ensuring safety in patients with co-existing autoimmune RDs. Thus, we investigated the incidence and severity of disease flares of pre-existing RDs, with a focus on rheumatoid arthritis (RA), in patients with solid tumors treated with ICIs.
Methods: A systematic review was conducted in PubMed/MEDLINE and Embase for all peer-reviewed articles from inception to May 16, 2024. Eligible studies were cohort studies and case series ( >5 cases) involving patients with solid tumors and concomitant RD treated with ICI therapy. A proportional meta-analysis with a random-effects model was conducted to pool the incidence of grade 1-5 flares and grade 3-5 severe flares in RDs and RA based on Common Terminology Criteria for Adverse Events (CTCAE) across the included studies.
Results: Among 2599 studies, 247 articles were eligible for full-text review, and 31 studies were included in the analysis. In total, 579 patients with RA, systemic lupus erythematosus, Sjögren’s syndrome, systemic sclerosis, sarcoidosis, spondyloarthritis, myositis, Behçet’s disease, vasculitis, polymyalgia rheumatica were included. Three studies evaluated PD-1/PD-L1 inhibitor monotherapy, two studies evaluated CTLA-4 inhibitor monotherapy, and 12 studies evaluated dual ICIs (ICI subtype unclear in 14 studies). Among all the RDs, the pooled incidence of flares was 33.8% (95% CI, 25.8–42.2; Figure 1). The median time to the first flare after initiating ICIs was 38.5 days (interquartile range: 30.0 – 52.6 days). The incidence of severe flares among overall RDs was 4.2% (95% CI, 0.6-9.6). Patients with RA were included in 22 studies with 343 patients. The pooled incidence of flares in patients with RA was 40.9% (95% CI, 32.2–49.8; Figure 2). Among patients with RA who developed flares, the incidence of severe flares was 8.3% (95% CI, 4.3–15.4).
Conclusion: Our study revealed that more than 30% of cases with RDs and RA develop flare with ICI therapy. These findings would be informative upon risk and benefit discussion before the initiation of ICI therapy for patients with solid tumors and concurrent RDs. Future studies are warranted to explore risk factors of flare and preventative strategies to mitigate the risk of flare development.
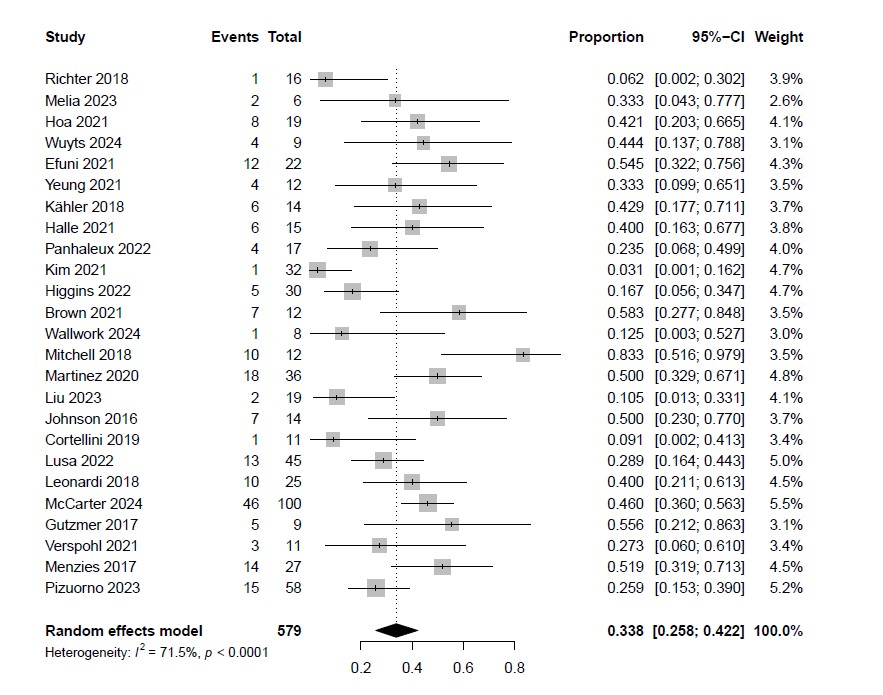 Figure 1: Incidence of flares in patients with pre-existing RDs
Figure 1: Incidence of flares in patients with pre-existing RDs
.jpg) Figure 2: Incidence of flares in patients with pre-existing RA
Figure 2: Incidence of flares in patients with pre-existing RA
To cite this abstract in AMA style:
Matsui T, Yamada K, Takahashi T, Nishimura Y, Fujiwara Y. Incidence of Flares in Pre-existing Rheumatologic Diseases Among Patients with Solid Tumors Undergoing Cancer Immunotherapy: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/incidence-of-flares-in-pre-existing-rheumatologic-diseases-among-patients-with-solid-tumors-undergoing-cancer-immunotherapy-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-flares-in-pre-existing-rheumatologic-diseases-among-patients-with-solid-tumors-undergoing-cancer-immunotherapy-a-systematic-review-and-meta-analysis/
