Session Information
Date: Tuesday, October 28, 2025
Title: Plenary III (1722–1727)
Session Type: Plenary Session
Session Time: 8:15AM-8:30AM
Background/Purpose: Many systemic autoimmune diseases associated with chronic type 1 interferon (IFN) signaling, including SLE, SjD, and SSc, preferentially afflict females. The biological basis of this female sex bias and its role in chronic IFN signaling are not well understood. Emerging data have implicated the X chromosome as a key contributor to female-biased autoimmunity. In XX individuals, X chromosome dosage is balanced with that of XY individuals via X-Chromosome Inactivation (XCI), an epigenetic mechanism that transcriptionally silences supernumerary X chromosomes via the long non-coding RNA, XIST. Strikingly, T and B cells from females with SLE exhibit epigenetic features of impaired XCI maintenance (XCIm). However, it is unclear from these data whether impaired XCIm in T or B cells directly contributes to IFN-mediated disease. Given the many immunomodulatory X-linked genes with roles in lymphocyte function and IFN signaling, we investigated whether impaired XCIm in female lymphocytes modifies the development of IFN-driven systemic autoimmunity.
Methods: We generated female C57BL/6 mice with either a B-cell-specific deletion of Xist (Mb1cre+/-Xistfl/fl, “Xist BcKO”) or a T cell-specific deletion of Xist (Cd4cre+Xistfl/fl, “Xist TcKO”). Female Xist BcKO mice and littermate controls (“WT”) or female Xist TcKO mice and littermate controls (“WT”) were treated thrice weekly with topical imiquimod (IMQ), a Tlr7 agonist, for 12 weeks (Fig. 1A). Serum, spleens, cervical lymph nodes, kidneys, and other tissues were harvested upon euthanasia and processed for autoantibody quantification, flow cytometry, and histology.
Results: IMQ-treated Xist BcKO mice exhibited markedly increased spleen mass, increased anti-dsDNA and anti-U1RNP/Smith autoantibody production, and more severe glomerular disease compared to WT mice (Fig. 1B-C). In contrast, IMQ-treated Xist TcKO mice did not exhibit significant differences in spleen mass, autoantibody levels, or glomerular pathology compared to IMQ-treated WT mice (Fig. 1D). Unlike IMQ-treated Xist TcKO mice, IMQ-treated Xist BcKO mice exhibited greater proportions of antibody-secreting and activated T and B cell subsets, including germinal center (GC) and age-associated B cells (ABCs), across the spleen, lymph nodes, and peripheral blood. Anti-U1RNP/Smith autoantibody levels were strongly correlated with the proportion of ABCs across all tissues; both rely on Tlr7 signaling (Fig. 2A-B). Activated B cell subsets from IMQ-treated Xist BcKO mice exhibited higher median fluorescence intensities of X-linked proteins, including Tlr7 and Cxcr3, suggesting their aberrant overexpression in the setting of Xist deletion (Fig. 2C).
Conclusion: These data demonstrate that impaired XCIm via Xist deletion has cell-type-specific effects on IFN-driven autoimmunity. They also demonstrate that impaired XCIm in B cells, but not T cells, amplifies humoral, cellular, and histopathological features of IFN-driven disease, and results in the overexpression of X-linked proteins likely derived from the inactive X chromosome. These findings support a novel mechanism of female-biased autoimmunity wherein impaired XCIm in B cells enhances susceptibility to IFN-mediated autoimmunity.
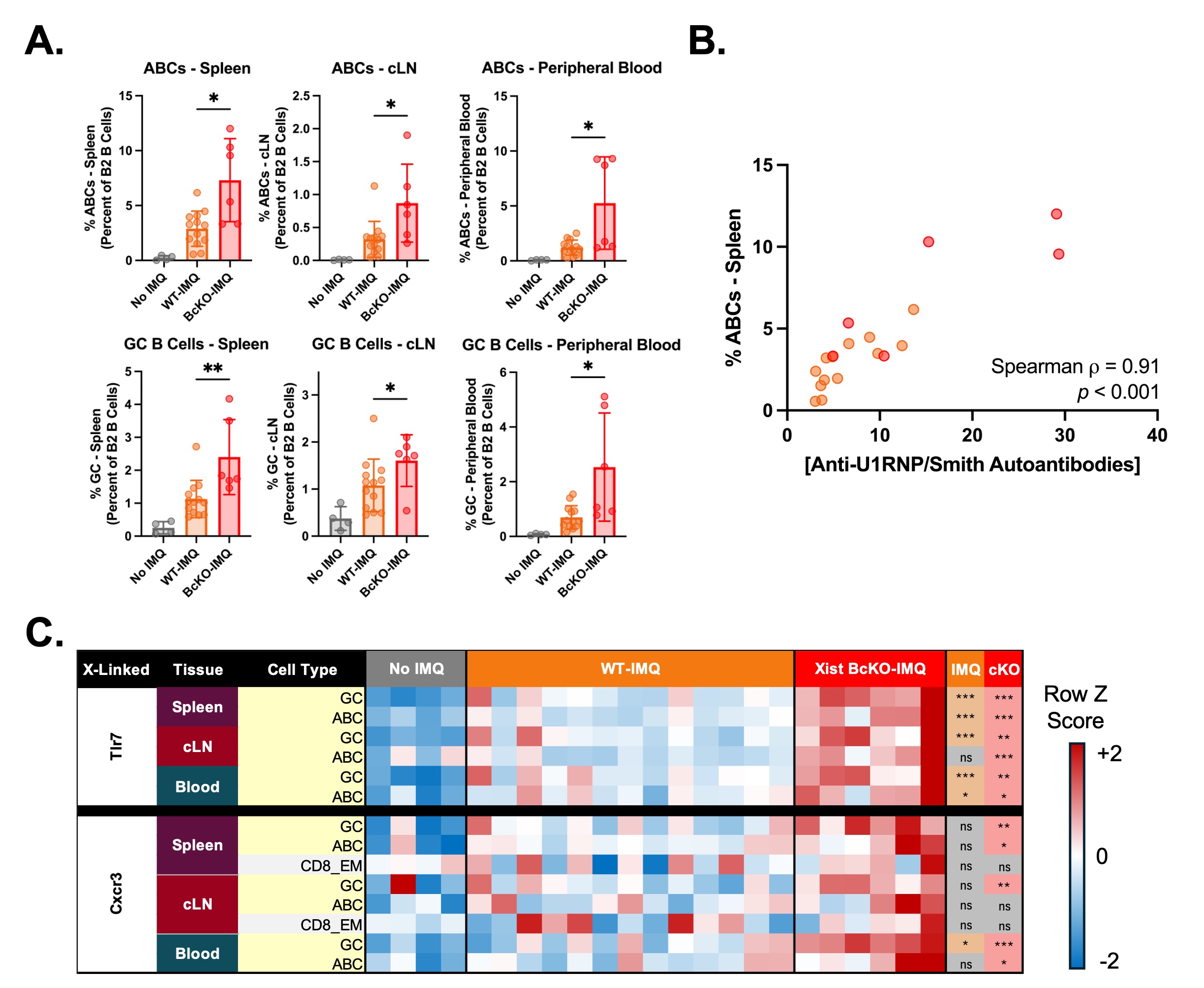 Figure 1. Xist Deletion in B Cells, But Not T Cells, Exacerbates Humoral and Histopathological Features of IMQ-Induced Disease. A. Schematic of the experimental approach. B. Quantification of relative spleen mass, autoantibody concentration, and glomerular pathology in IMQ-treated Xist BcKO mice (n=7) versus IMQ-treated WT mice (n= 13). The “Glomerular Pathology Score” represents the sum of subscores (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe) for key histopathologic features specific to the glomerulus; 10-50% of glomeruli were affected in most mice. C. Images of an unaffected and a diseased glomerulus (40X; same image scale), stained with periodic acid-Schiff (PAS). D. Quantification of relative spleen mass, autoantibody concentration, and glomerular pathology in IMQ-treated Xist TcKO mice (n=16) versus IMQ-treated WT mice (n=21). WT mice that were not treated with IMQ are included for both experiments to serve as a reference (n=4 for Xist BcKO experiments; n=12 for Xist TcKO experiments). Data are depicted as mean +/- SD. *, **, *** = p < 0.05, 0.01, 0.001 via pairwise Mann-Whitney U test (IMQ-cKO vs. IMQ-WT). ns = not statistically significant; Xa = active X chromosome; Xi = inactive X chromosome.
Figure 1. Xist Deletion in B Cells, But Not T Cells, Exacerbates Humoral and Histopathological Features of IMQ-Induced Disease. A. Schematic of the experimental approach. B. Quantification of relative spleen mass, autoantibody concentration, and glomerular pathology in IMQ-treated Xist BcKO mice (n=7) versus IMQ-treated WT mice (n= 13). The “Glomerular Pathology Score” represents the sum of subscores (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe) for key histopathologic features specific to the glomerulus; 10-50% of glomeruli were affected in most mice. C. Images of an unaffected and a diseased glomerulus (40X; same image scale), stained with periodic acid-Schiff (PAS). D. Quantification of relative spleen mass, autoantibody concentration, and glomerular pathology in IMQ-treated Xist TcKO mice (n=16) versus IMQ-treated WT mice (n=21). WT mice that were not treated with IMQ are included for both experiments to serve as a reference (n=4 for Xist BcKO experiments; n=12 for Xist TcKO experiments). Data are depicted as mean +/- SD. *, **, *** = p < 0.05, 0.01, 0.001 via pairwise Mann-Whitney U test (IMQ-cKO vs. IMQ-WT). ns = not statistically significant; Xa = active X chromosome; Xi = inactive X chromosome.
.jpg) Figure 2. IMQ-treated Xist BcKO Mice Exhibit Increased Proportions of Activated B Cell Subsets in the Setting of Increased Tlr7 Protein Levels. A. Proportions of activated B cell subsets across the spleen, cervical lymph nodes, and peripheral blood in untreated WT, IMQ-treated WT, and IMQ-treated Xist BcKO mice, quantified via flow cytometry‡. Data are depicted as mean +/- SD. *, ** = p < 0.05, and 0.01, respectively, via pairwise Mann-Whitney U test (BcKO-IMQ vs. WT-IMQ). B. Scatterplot and Spearman correlation of anti-U1RNP/Smith antibody concentration versus the percent of splenic age-associated B cells among IMQ-treated mice. Data are colored by experimental group. C. Heatmap depicting the row-normalized median fluorescence intensities of Tlr7 and Cxcr3 across B cell subsets, tissue types, and experimental groups. Each column represents a unique biological sample. The results of the pairwise Mann-Whitney U tests are depicted in the columns “IMQ” and “cKO”. “IMQ” refers to the pairwise comparison between “WT-IMQ” and “No IMQ”. “CKO” refers to the pairwise comparison between “BcKO-IMQ” and “WT-IMQ”. *, **, *** = p < 0.05, 0.01, and 0.001, respectively. ABCs = age-associated B cells (Cd21/35-Cd23-Cd11c+Tlr7+); CD8_EM = CD8+ Effector Memory T cells (Cd62l-Cd44+); cLN = cervical lymph nodes; GC = germinal center B cells (Gl7+Cd95+). ‡ = n=1 IMQ-treated Xist BcKO mouse excluded from cytometry analyses due to death 48 hours prior the experimental endpoint.
Figure 2. IMQ-treated Xist BcKO Mice Exhibit Increased Proportions of Activated B Cell Subsets in the Setting of Increased Tlr7 Protein Levels. A. Proportions of activated B cell subsets across the spleen, cervical lymph nodes, and peripheral blood in untreated WT, IMQ-treated WT, and IMQ-treated Xist BcKO mice, quantified via flow cytometry‡. Data are depicted as mean +/- SD. *, ** = p < 0.05, and 0.01, respectively, via pairwise Mann-Whitney U test (BcKO-IMQ vs. WT-IMQ). B. Scatterplot and Spearman correlation of anti-U1RNP/Smith antibody concentration versus the percent of splenic age-associated B cells among IMQ-treated mice. Data are colored by experimental group. C. Heatmap depicting the row-normalized median fluorescence intensities of Tlr7 and Cxcr3 across B cell subsets, tissue types, and experimental groups. Each column represents a unique biological sample. The results of the pairwise Mann-Whitney U tests are depicted in the columns “IMQ” and “cKO”. “IMQ” refers to the pairwise comparison between “WT-IMQ” and “No IMQ”. “CKO” refers to the pairwise comparison between “BcKO-IMQ” and “WT-IMQ”. *, **, *** = p < 0.05, 0.01, and 0.001, respectively. ABCs = age-associated B cells (Cd21/35-Cd23-Cd11c+Tlr7+); CD8_EM = CD8+ Effector Memory T cells (Cd62l-Cd44+); cLN = cervical lymph nodes; GC = germinal center B cells (Gl7+Cd95+). ‡ = n=1 IMQ-treated Xist BcKO mouse excluded from cytometry analyses due to death 48 hours prior the experimental endpoint.
To cite this abstract in AMA style:
Jiwrajka N, Lovell C, Searcy Z, Forsyth K, Welter E, Toothacre N, Valero-Pacheco N, Premo K, Anguera M. Impaired Maintenance of X-Chromosome Inactivation in B Cells, But Not T Cells, Exacerbates Interferon-Driven Systemic Autoimmunity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impaired-maintenance-of-x-chromosome-inactivation-in-b-cells-but-not-t-cells-exacerbates-interferon-driven-systemic-autoimmunity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impaired-maintenance-of-x-chromosome-inactivation-in-b-cells-but-not-t-cells-exacerbates-interferon-driven-systemic-autoimmunity/
