Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Rheumatoid Arthritis – Treatment I: Preventative and Novel Treatments (1674–1679)
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: Clinical trials aimed at delaying or preventing rheumatoid arthritis (RA) in individuals at risk have been published with variable results. In the APIPPRA study, 52 weeks of treatment with abatacept reduced rates of progression to RA, effects sustained beyond the treatment period. The aim of this study was to capture long term outcomes in the ALTO study to establish whether one year of abatacept treatment prevents or delays the onset of RA in subsequent years.
Methods: APIPPRA was a phase IIB randomised, controlled trial recruiting 213 ACPA+- individuals with inflammatory arthralgia but no clinical synovitis. Participants were randomised to receive 52 weekly subcutaneous injections of placebo (n=103) or 125mg abatacept (n=110) and followed up for a further 52 weeks. All study participants deemed eligible and randomised to the APIPPRA study were invited to participate in the follow up study. The primary endpoint was the time to development of either clinical synovitis in ³ 3 joints, RA according to ACR/EULAR 2010 criteria, or time to first treatment with disease modifying anti-rheumatic drugs, whichever was met first. Primary endpoints were evaluated after stratifying for serum autoantibodies defined at the APIPPRA baseline visit. Secondary endpoints included multiple disease activity assessments. Severe adverse events and events of special interest were recorded.
Results: Between 26th April 2021 and 31st January 2023, 143 enrolled in ALTO; 71 randomised to abatacept and 72 to placebo. Median follow up time was 66 months (Q25-Q75: 54 – 78). At the end of follow up, there were 119 primary outcome events. By the end of the first year after randomisation, the cumulative Kaplan-Meier proportion with arthritis was 29% in the placebo arm and 6% in the abatacept arm. At the end of 2 years these were 40% and 30%, and at the end of the third and fourth years, differences between arms were 4% and 2% respectively (Figure 1A). Differences in restricted mean arthritis-free survival time up to 2 years were 3.3 months (95% CI 1.4 – 5.3; p=0.001), up to 3 years 4.4 months (95% CI 1.1 – 7.8; p=0.008), and up to 4 years 5.0 months (95% CI 0.38 – 9.8; p=0.039). Differences were no longer sustained at 5 years. Stratifying by baseline IgG ACPA levels the cumulative proportion with arthritis after 2 years was 49% in the placebo arm compared to 27% in the abatacept arm in those with levels 340 IU/ml; by 4 years it was 67% and 59% for placebo and abatacept, respectively. For those with an extended serotype (IgG and IgA ACPA, anti-carbamylated protein antibodies, anti-acetylated protein antibodies and RF, designated 5 serotypes), the cumulative proportion with arthritis was 53% for placebo and 12% for abatacept after 2 years, and 67% and 47% by the end of 4 years of follow up, differences sustained to 6 years (Figure 1B). There were 5 SAEs in those randomised to the abatacept arm and one in the placebo arm; none were deemed related to study drug.
Conclusion: Interception with abatacept for one year delays progression to RA for up to 4 years. Those at highest risk of progression have a more mature autoantibody response and are sensitive to T cell co-stimulation modulation. RA interception with abatacept is well tolerated, with no new safety signals emerging during follow up.
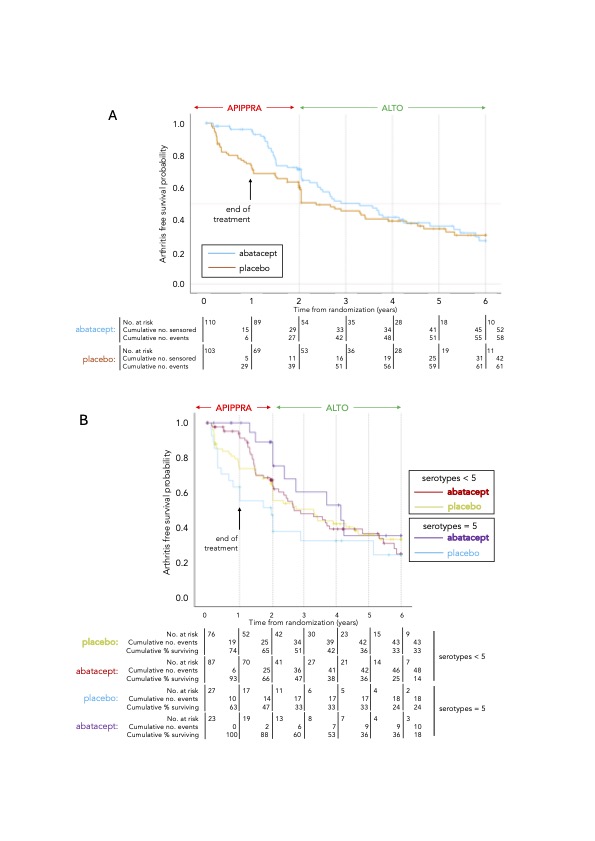 Figure 1: Kaplan-Meier arthritis-free survival curves by study arm for the combined APIPPRA and ALTO study periods for the intention to treat population (A) and stratified by baseline autoantibody status (B).
Figure 1: Kaplan-Meier arthritis-free survival curves by study arm for the combined APIPPRA and ALTO study periods for the intention to treat population (A) and stratified by baseline autoantibody status (B).
To cite this abstract in AMA style:
Cope A, Vasconcelos J, Jasenecova M, Filer A, Raza K, Qureshi S, D'Agostino M, McInnes I, Siebert S, Isaacs J, Pratt A, Fisher B, Buckley C, Emery P, Mankia K, Ho P, BUCH M, Ciurtin C, van Schaardenburg D, Huizinga T, Toes R, Murphy C, Prevost A. Abatacept in individuals at risk of developing rheumatoid arthritis: results from the Arthritis Prevention in the Pre-clinical Phase of RA with Abatacept Long Term Outcomes (ALTO) Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/abatacept-in-individuals-at-risk-of-developing-rheumatoid-arthritis-results-from-the-arthritis-prevention-in-the-pre-clinical-phase-of-ra-with-abatacept-long-term-outcomes-alto-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-in-individuals-at-risk-of-developing-rheumatoid-arthritis-results-from-the-arthritis-prevention-in-the-pre-clinical-phase-of-ra-with-abatacept-long-term-outcomes-alto-study/
