Session Information
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: The gut-joint and mucosal origins hypotheses postulate that immune alterations in mucosal sites may precede and impact the development of rheumatoid arthritis and spondyloarthritis. However, the mechanisms behind these connections remain unknown. Our prior work using KikGR transgenic mice, in which we performed colonoscopy-guided green-to-red photoconversion, demonstrated that colon intraepithelial lymphocytes (cIELs) constitutively traffic. The trafficking cIELs, referred to as gut-derived T cells, were characterized as TCRαβ+ CD4+ and found in extraintestinal tissues including the spleen, liver, lungs, and joints. In this study, we aimed to further elucidate the functions of gut-derived T cells and the intestinal signals that influence their systemic function.
Methods: Using colonoscopy-guided photoconverted KikGR mice, we performed single cell RNA sequencing of sorted KikGreen+ (non-gut-derived) and KikRed+ (gut-derived) splenocytes, then validated results by flow cytometry. We used an MHCII tetramer containing the flagellin peptide Cbir1, a common T cell antigenic target, to evaluate broad commensal reactivity. We then generated KikGR+ I-Abfl/fl Villin-Cre (MHCIIΔΙΕC) mice, which lack expression of MHCII on intestinal epithelial cells, and performed single cell ATAC sequencing on cIELs from MHCIIΔIEC and MHCIIWT mice followed by flow cytometry on trafficking cells.
Results: Differential gene expression analysis of our RNA sequencing data revealed increased transcription of Cd52, a marker of regulatory function, and decreased transcription of the AP-1 transcription factor family members Fos, Jun, and Maf in gut-derived CD4+ T cells, suggesting either antigen inexperience or anergy. Flow cytometry of lamina propria, spleen, mesenteric lymph nodes, and joint-draining lymph nodes confirmed significantly higher numbers of gut-derived regulatory CD52high and anergic CD73+ FR4+ CD4+ T cells and significantly lower numbers of gut-derived naïve CD62L+ CD44- T cells. Cbir1-reactive CD4+ T cells, representative of microbiome reactivity, were more frequent in gut-derived than non-gut-derived cells, and gut-derived Cbir1-reactive T cells expressed more CD73 and FR4 as well as CD52 than non-gut-derived Cbir1-reactive cells. We then hypothesized that epithelial antigen presentation to commensal-reactive cIELs induced anergy. Single cell ATAC sequencing of MHCIIΔIEC cIELs revealed modest decreases in accessibility of NFAT binding sites compared to cIELs from littermate controls. However, we did not see significant differences in expression of regulatory markers (Foxp3, CD73 and FR4, CD52) or antigen experience markers (CD62L, CD44) by flow cytometry.
Conclusion: Our analysis of gut-derived CD4+ T cells suggest that these cells are commensal-reactive and may play a role in peripheral tolerance through induction of anergy or expression of the regulatory marker CD52. These data are in line with our previous work demonstrating that gut-derived T cells help resolve joint inflammation. Ongoing studies will now assess the role of intestinal inflammation on gut-derived T cell function as well as interrogate how these cells gain their function.
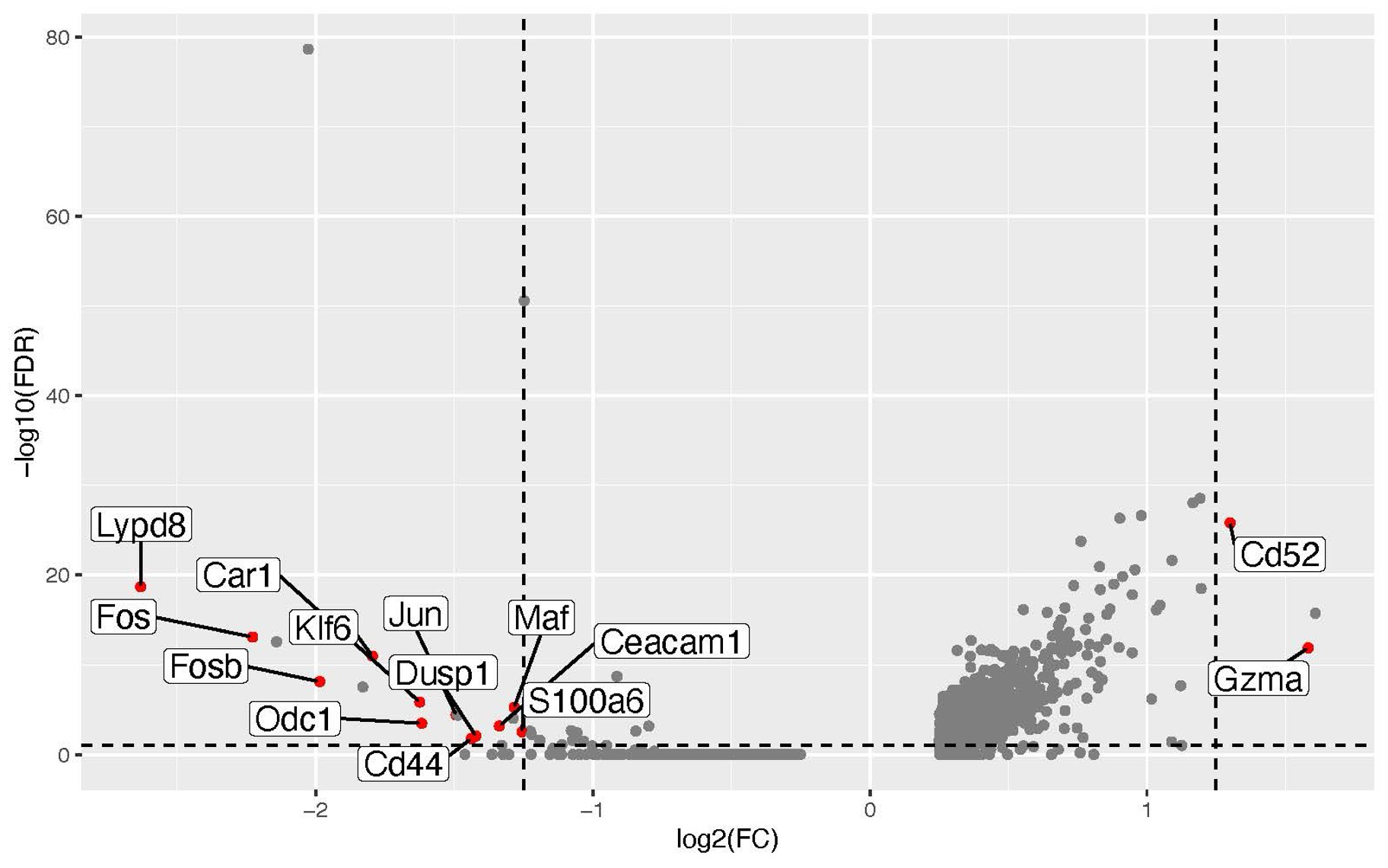 Volcano plot of differentially expressed genes in gut-derived CD4+ splenocytes after RNA sequencing.
Volcano plot of differentially expressed genes in gut-derived CD4+ splenocytes after RNA sequencing.
.jpg) cIELs and cells from spleen (A) or joint-draining lymph nodes (B) were isolated from colonoscopy-guided photoconverted KikGR mice and expression of the indicated markers was evaluated CD4+ TCRαβ+ T cells from each tissue by flow cytometry. Non-gut-derived and gut-derived cells were compared using multiple Wilcoxon tests. *, p < 0.05; **, p < 0.01.
cIELs and cells from spleen (A) or joint-draining lymph nodes (B) were isolated from colonoscopy-guided photoconverted KikGR mice and expression of the indicated markers was evaluated CD4+ TCRαβ+ T cells from each tissue by flow cytometry. Non-gut-derived and gut-derived cells were compared using multiple Wilcoxon tests. *, p < 0.05; **, p < 0.01.
To cite this abstract in AMA style:
Danielson S, Liu S, Kuhn K. Gut-joint lymphocyte trafficking functions to regulate systemic immunity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/gut-joint-lymphocyte-trafficking-functions-to-regulate-systemic-immunity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gut-joint-lymphocyte-trafficking-functions-to-regulate-systemic-immunity/
