Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Global assessments by patients and physicians provide unique but complementary perspectives of disease severity. This study aimed to determine the clinical and patient-reported factors associated with patient and physician global assessments in systemic sclerosis.
Methods: Adult patients with early systemic sclerosis (SSc) (< 5 years from first non-Raynaud’s phenomenon symptom) from the COllaborative National QUality and Efficacy Registry (CONQUER) were included. Global disease assessments by patients (PtGA) and physicians (PhGA)(0-10, higher is worse, 7-day recall), clinical evaluations, and patient-reported outcomes were collected at enrollment. Multivariable linear regression models determined factors associated with each global assessment using i) pre-specified clinical variables only (Model 1), and ii) clinical variables plus patient-reported outcomes (Model 2). Relative weight analysis (RWA), an approach to evaluate variable importance among correlated variables, determined the relative importance of each factor as a portion of the total variance explained by the models.
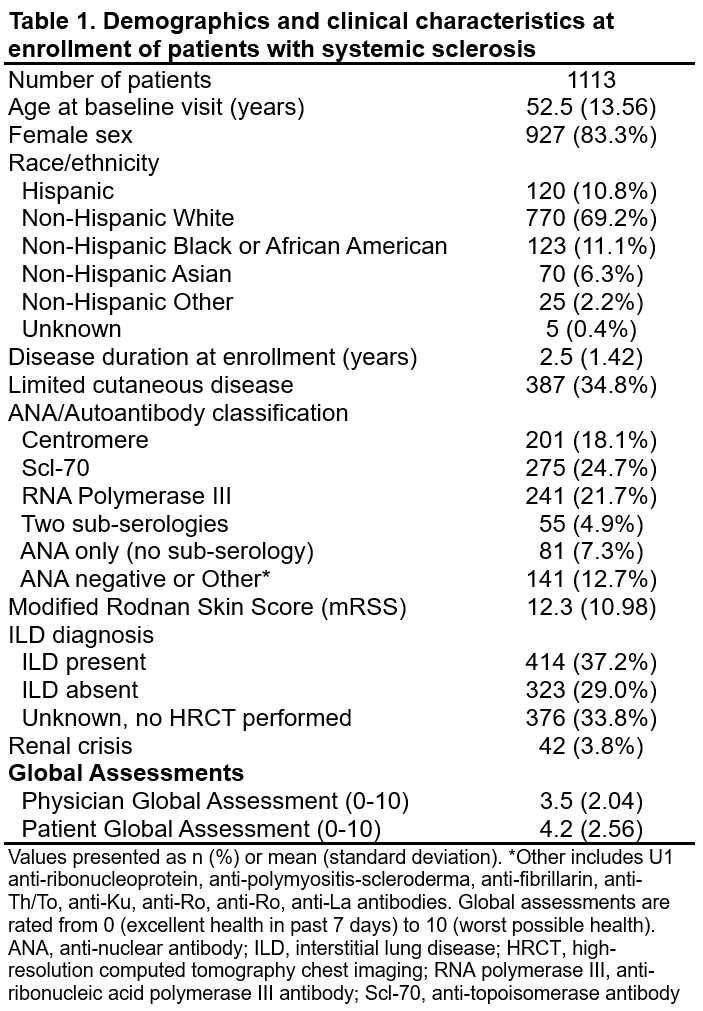
Results: Data from 1113 patients (83% female, 35% limited cutaneous disease, mean (standard deviation, SD) disease duration 2.5 (1.4) years) are included (Table 1). Mean (SD) global assessment scores at enrollment were 4.2 (2.6) and 3.5 (2.0) among patients and physicians, respectively (p< 0.001). Clinical variables explained 20% of the variability in the PtGA and 42% of the variability in the PhGA. Clinical and patient-reported variables explained 39% of the variability in the PtGA and 49% of the variability in the PhGA. RWA from Model 1 identified modified Rodnan skin score (mRSS) and New York Heart Association (NYHA) Function Class as clinical variables with the highest importance for both PtGA and PhGA (Figure 1a, 1b). RWA from Model 2 demonstrated a shift in the most important contributors to PtGA from clinical measures to patient-reported measures including overall pain/discomfort (16.1%), PROMIS physical function (9.7%), PROMIS Pain Interference (9.4%), Patient Skin Assessment (8.7%), and PROMIS Social Roles (6.9%) (Figure 2a). For the PhGA, RWA based on Model 2 demonstrated that mRSS (13.1%), NYHA Class III/IV (7.2%), Patient Skin Assessment (6.5%), diffusing capacity of the lung for carbon monoxide (DLCO) %-predicted (5.4%), and overall pain/discomfort (5.0%) had the highest importance (Figure 2b).
Conclusion: Factors associated with global assessments in early SSc differ between patients and physicians. Measures of skin involvement and functional status are important contributors to both PtGA and PhGA. However, the PtGA is most influenced by health-related quality of life symptoms that are not well-captured with traditional disease measures. The PhGA is influenced more heavily by traditional clinical factors, but physicians may underestimate the impact of patients’ symptom burden. These findings highlight the broad impact of patients’ experiences and the importance of collecting both patient- and clinician-reported outcomes in research in SSc.
.jpg) Figure 1. Relative weights of clinical variables associated with the patient and physician global assessment of disease in systemic sclerosis.
Figure 1. Relative weights of clinical variables associated with the patient and physician global assessment of disease in systemic sclerosis.
Relative weight analysis (RWA) using enrollment visit data determined the relative importance of clinical variables (Model 1) associated with the a) patient global assessment and b) physician global assessment. Relative weights (%) indicate the proportion of variance explained by the variable out of the total variance explained by the model. Plots depict the 20 variables with the highest relative weights. For the patient global, R-squared = 0.20 and the 20 variables shown account for 74% of the total variance. For the physician global, R-squared = 0.42 and the 20 variables shown account for 90% of the total variance. Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NYHA, New York Heart Association; PROMIS, Patient-reported outcomes measurement information system; RNA polymerase III, anti-ribonucleic acid polymerase III antibody; Scl-70, anti-topoisomerase antibody; SHAQ, Scleroderma Health Assessment Questionnaire; VAS, visual analogue scale.
.jpg) Figure 2. Relative weights of clinical and patient-reported variables associated with the patient and physician global assessments of disease in systemic sclerosis.
Figure 2. Relative weights of clinical and patient-reported variables associated with the patient and physician global assessments of disease in systemic sclerosis.
Relative weight analysis (RWA) using enrollment visit data determined the relative importance of clinical and patient-reported variables (Model 2) associated with the a) patient global assessment and b) physician global assessment. Plots depict the 20 variables with the highest relative weights. Patient-reported variables are indicated by striped bars. For the patient global, R-squared = 0.39 and the 20 variables shown account for 90% of the total variance. For the physician global, R-squared = 0.49 and the 20 variables shown account for 79% of the total variance. Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NYHA, New York Heart Association; PROMIS, Patient-reported outcomes measurement information system; RNA polymerase III, anti-ribonucleic acid polymerase III antibody; Scl-70, anti-topoisomerase antibody; SHAQ, Scleroderma Health Assessment Questionnaire; VAS, visual analogue scale.
To cite this abstract in AMA style:
Romich E, Ogdie A, Merkel P, Stephens Shields A, Alvey J, Assassi S, Bernstein E, Bracken S, Castelino F, Chung L, Evnin L, Frech T, Gordon J, Hant F, Harding M, Hummers L, Khanna D, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Mayes M, McMahan Z, Molitor J, Moore D, Richardson C, Shah A, Shah A, Skaug B, Steen V, VanBuren J, Volkmann E, Zahn C, Sandorfi N. Factors Associated with Patient and Physician Global Assessments in Early Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-patient-and-physician-global-assessments-in-early-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-patient-and-physician-global-assessments-in-early-systemic-sclerosis/