Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti- Cytolethal Distending Toxin (CDT) antibodies may serve as biomarkers for post-infectious autoimmunity and aid in clinical risk stratification. In this study, we aimed to determine the prevalence of anti-CDT antibodies in a large, well-characterized cohort of patients with systemic sclerosis (SSc) and to examine associations with gastrointestinal (GI) and extraintestinal clinical features and other SSc-related antibodies.
Methods: Sera from 130 well-characterized patients with SSc enriched for GI disease were screened for anti-CDT antibodies by ELISA. Clinical features, UCLA GIT 2.0-assessed symptoms, and autoantibody profiles were compared between patients with and without anti-CDT antibodies.
Results: Anti-CDT antibodies were detected in 18% (23/130) of SSc patients. Patients with anti-CDT antibodies more frequently had significant GI disease (87% vs. 71%; p=0.19) and were significantly more likely to have antibodies to U11/U12 (RNPC3) (35% vs. 4%; p < 0.001), U1RNP (26% vs. 8%; p=0.018), and topoisomerase-1 (30% vs. 12%; p=0.044). UCLA GIT Soilage scores were significantly higher (worse) among anti-CDT -positive patients [1 (1, 2) vs. 0 (0, 2); p=0.037]. In multivariable analyses, anti-CDT antibodies remained significantly associated with anti-U11/U12 (RNPC3) [OR 19.0 (4.3, 83.4); p= < 0.001), anti-U1RNP antibodies [OR 7.2 (1.9, 27.9); p=0.004] and anti-topoisomerase-1 antibodies [OR 4.7 (1.3, 16.8); p=0.019], even after adjusting for potential confounders.
Conclusion: Anti-CDT antibodies may serve as markers of post-infectious autoimmunity and are strongly associated with SSc-specific autoantibodies linked to severe, progressive GI disease. Understanding the connection between anti-CDT antibodies and autoimmune mechanisms may provide insight into disease pathogenesis.
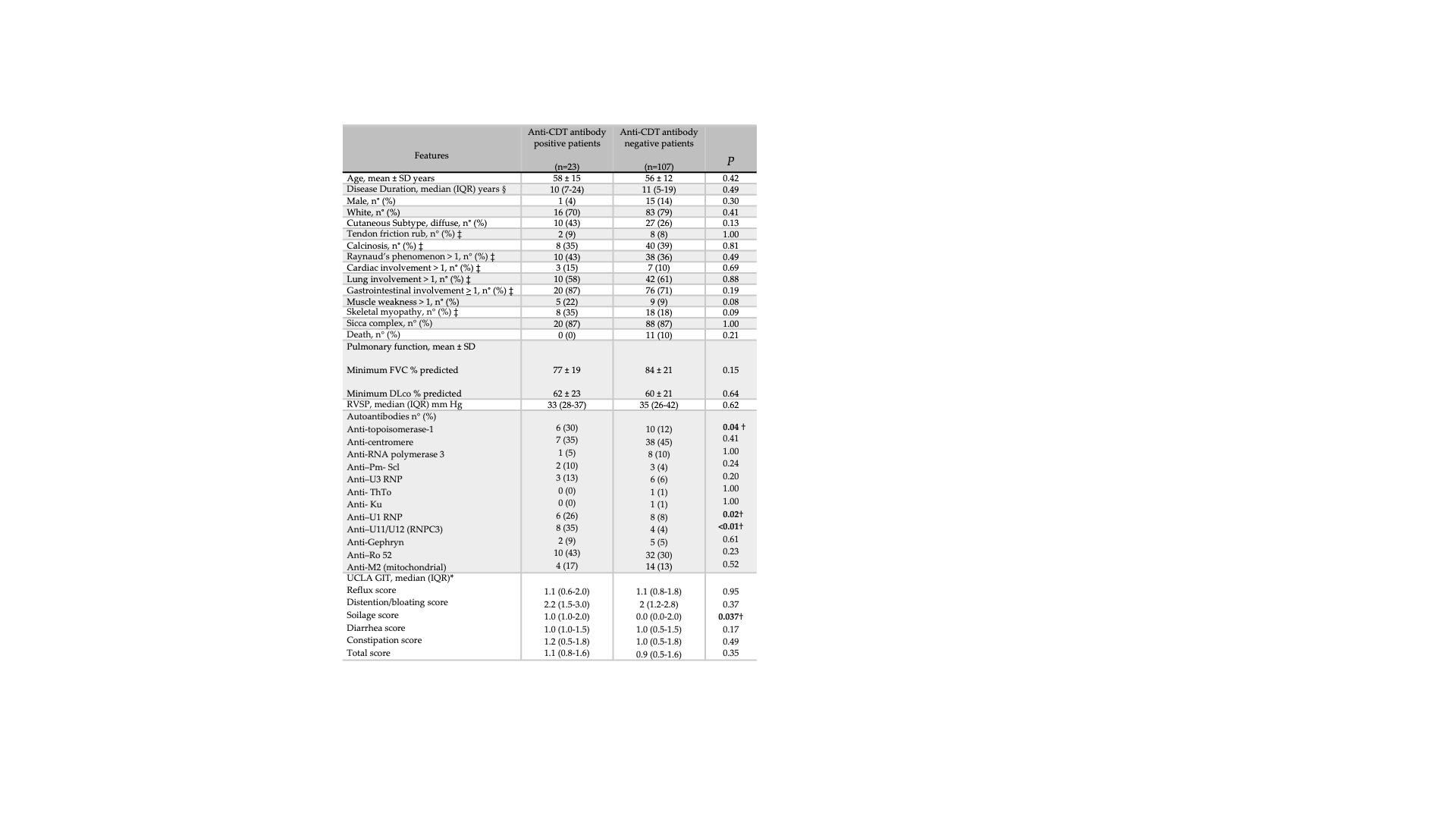 Table 1. Comparison of demographic, clinical and serological features between anti-CDT antibody positive and negative patients with systemic sclerosis.
Table 1. Comparison of demographic, clinical and serological features between anti-CDT antibody positive and negative patients with systemic sclerosis.
SD= standard deviation; IQR = interquartile range; FVC = forced vital capacity; DLco =diffusing capacity for carbon monoxide; RVSP = right ventricular systolic pressure; p= p-value.
§ Disease duration from any first symptom to the date of the serum sample collection.
‡ Maximum Medsger severity score ever recorded in the database; FVC and DLco are represented by the minimum values ever recorded.
*UCLA GIT 2.0, University of California Los Angeles Scleroderma Clinical Trials Consortium gastrointestinal tract 2.0.
† Statistically significant.
.gif) Table 2. Statistical models assessing the association between anti–CDT–positive patients with systemic sclerosis and clinical, serological features.
Table 2. Statistical models assessing the association between anti–CDT–positive patients with systemic sclerosis and clinical, serological features.
† Statistically significant.
*Values are odds ratio (95% confidence interval).
To cite this abstract in AMA style:
Di Ciommo F, Casciola-Rosen L, Balar A, Shah A, Hughes M, Morales W, Pimentel M, Adler B, McMahan Z. Anti-Cytolethal Distending Toxin Antibodies in Systemic Sclerosis: Associations with Gastrointestinal Disease and Immune Dysregulation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-cytolethal-distending-toxin-antibodies-in-systemic-sclerosis-associations-with-gastrointestinal-disease-and-immune-dysregulation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-cytolethal-distending-toxin-antibodies-in-systemic-sclerosis-associations-with-gastrointestinal-disease-and-immune-dysregulation/
