Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Inflammatory arthritis is a cardinal manifestation of systemic lupus erythematosus (SLE), limiting joint mobility and adversely affecting quality of life.1 Joint activity in SLE can be quantified using Tender Joint Counts and Swollen Joint Counts, providing concrete data for the evaluation of treatment effectiveness.2 Clinical trials have shown reductions in swollen and tender joint counts in patients treated with the type I interferon inhibitor anifrolumab, compared to placebo.3 In this study, we assess changes in inflammatory arthritis before and after anifrolumab initiation among adults with SLE receiving treatment in real-world settings.
Methods: This retrospective, observational cohort study included data from adult patients with SLE in the American Rheumatology Network initiating anifrolumab (index date) from August 2, 2021 to May 31, 2023. Eligible patients adhered to anifrolumab, defined as having 6 or more infusions in a 12-month period. Tender and Swollen Joint Counts (28 joints) were captured during 6-month intervals: 6 months pre-index (baseline); 0-6 months post-index (follow-up Period 1); >6-12 months post-index (follow-up Period 2). All patients were required to have at least one joint count assessment performed in each of the 6-month intervals, with the result closest to the end of each interval being assessed. Inflammatory arthritis (joint activity) was defined as having Tender Joint Counts or Swollen Joint Counts ≥1.
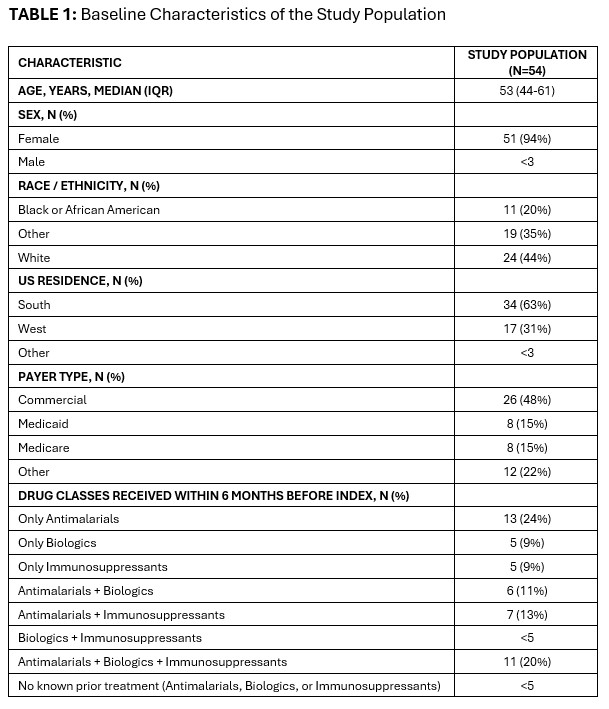
Results: Of the 54 included participants, 94% were female and 44% white [TABLE 1]. The median age was 53 years (IQR 44-61). A declining number of patients had swollen joints (Baseline: 54%; Period 1: 46%; Period 2: 41%) and joint tenderness (Baseline: 69%; Period 1: 61%; Period 2: 56%) during the first year of anifrolumab therapy [TABLE 2]. Joint tenderness (37/54) occurred more frequently than joint swelling (29/54) at baseline [TABLE 3]. In patients experiencing joint swelling prior to initiation of anifrolumab, the mean (SD) number of swollen joints progressively decreased from 9.5 (7.1) joints per patient in the Baseline period to 5.2 (5.7) in Period 2. Similarly, in patients with joint tenderness at baseline, the mean (SD) number of tender joints decreased from 5.4 (4.5) to 4.0 (4.6) joints per patient [TABLE 3]. After at least six months of receiving anifrolumab treatment, 34% (10/29) of patients with joint swelling and 27% (10/37) of those with joint tenderness at baseline had no joint activity.
Conclusion: In this real-world cohort, patients with SLE on anifrolumab therapy had marked improvement, with reductions in both swollen and tender joints observed within six months of treatment initiation. After at least six months of receiving anifrolumab treatment, approximately one-third of patients with active inflammatory arthritis became asymptomatic. These findings contribute to the growing body of evidence supporting improvement of inflammatory arthritis with anifrolumab therapy.1. Grossman JM. Best Pract Res Clin Rheumatol. 2009;23(4): 495-506.2. Bell CF, et al. Rheumatol Ther. 2023;10:261–74.3. Morand EF, et al. Lancet Rheumatol. 2022;4(4);e282-e292.
To cite this abstract in AMA style:
Kyttaris V, Persons D, Atefi G, Binka M. Improvement in Joint Activity in Adults with Systemic Lupus Erythematosus Treated with Anifrolumab: Results from a Real-World Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improvement-in-joint-activity-in-adults-with-systemic-lupus-erythematosus-treated-with-anifrolumab-results-from-a-real-world-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-joint-activity-in-adults-with-systemic-lupus-erythematosus-treated-with-anifrolumab-results-from-a-real-world-cohort/

.jpg)
.jpg)