Session Information
Date: Monday, October 27, 2025
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s disease (SjD) is a chronic autoimmune disease characterized by systemic symptoms such as dryness, fatigue, musculoskeletal pain, and extraglandular manifestations. SjD may overlap with other autoimmune diseases like systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). This analysis characterized the demographics, clinical characteristics, and patient-reported outcomes (PROs) of patients with SjD treated in a rheumatology setting and evaluated comorbidities 12 months post-diagnosis.
Methods: Adult patients in the Excellence Network in RheumatoloGY (ENRGY) rheumatology network with ≥2 ICD-9 and ICD-10-CM codes for SjD diagnoses ≥28 to ≤365 days apart from January 2017 through February 2023 were included in this retrospective analysis of electronic health record (EHR) data. Prior to diagnosis by a rheumatologist, patients were required to have ≥12 months without an SjD diagnosis. Patient EHR data were linked to administrative claims from the Komodo Health database. The index date was defined as the date of the first SjD diagnosis. Demographic and clinical data were captured at index. Clinical manifestations and PRO outcomes collected closest to and within 12 months before or after index were reported
Results: Among 2475 patients, 91.8% were female and the mean (SD) age was 52.9 (12.9) years. Dryness symptoms were common (55.8%) prior to diagnosis, including dry eyes (22.4%) and mouth (10.8%). Asthenia-polyalgia was the most commonly occurring symptom in the pre-index period (87.9%), including arthralgia (71.0%), fatigue (52.6%), or myalgia (23.0%) (Table 1). Many patients had ≥1 extraglandular symptom (70.7%) (Table 1). Among the 58.5% of patients who underwent SS-A antibody testing, 34.0% tested positive. Diagnostic procedures including salivary gland ultrasound (15.4%) were infrequent, and magnetic resonance sialography, ocular surface staining test, and unstimulated whole saliva flow test were rare (< 0.1%). Anxiety (35.9%) and depression (27.1%) were common, and 42.3% had overlapping autoimmune diseases pre- and post-index, such as RA (29.1%), SLE (15.6%), and systemic sclerosis (2.2%). Patient characteristics were similar irrespective of overlapping autoimmune disease (Table 1). Frequent comorbidities 12 months post-diagnosis included arthritis, anxiety, Raynaud’s disease, depression, and fibromyalgia (Figure 1). Mean (SD) PRO scores included EULAR Sjögren’s Syndrome Patient Reported Index (16.8 [6.5]; n=5), Routine Assessment of Patient Index Data 3 (10.5 [6.6]; n=1038), Pain 0-10 (4.5 [2.9]; n=1185), and Patient Global Assessment (2.4 [6.6]; n=1245).
Conclusion: This analysis of EHR data revealed a range of extraglandular symptoms and comorbidities with SjD. Diagnostic procedures were infrequent, and common symptoms like dryness were under-reported. PRO scores indicated high disease burden, with high rates of anxiety, depression, and arthritis—the latter most commonly co-occurring 1 year post-diagnosis. These findings reveal the complex clinical challenges faced by patients with SjD.
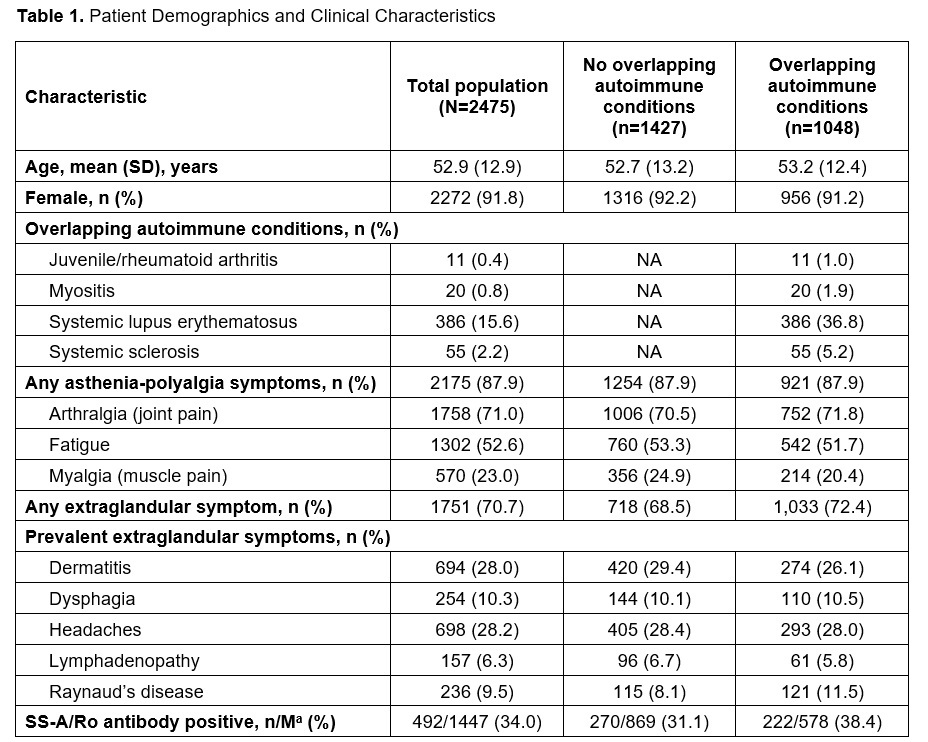 NA, not applicable; SS-A, Sjogren’s syndrome-related antigen A.
NA, not applicable; SS-A, Sjogren’s syndrome-related antigen A.
a n/M is the number of patients with positive test/number of evaluable patients.
.jpg) ESSPRI, EULAR Sjogren’s Syndrome Patient Reported Index; MDHAQ, Multidimensional Health Assessment Questionnaire; n/N, number of patients evaluated/total patient population; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; RAPID3, Routine Assessment of Patient Index Data 3; SjD, Sjögren disease; VAS, visual analog scale.
ESSPRI, EULAR Sjogren’s Syndrome Patient Reported Index; MDHAQ, Multidimensional Health Assessment Questionnaire; n/N, number of patients evaluated/total patient population; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; RAPID3, Routine Assessment of Patient Index Data 3; SjD, Sjögren disease; VAS, visual analog scale.
a Gray dotted lines represent cutoff points for moderate disease for each PRO.
b ESSPRI score of ≥5 indicates unsatisfactory symptom status.
c RAPID3 disease severity states are defined as remission (≤3), low (3.1-6), moderate (6.1-12), and high (≥12).
d MDHAQ physical function score of 3.3 to 6.6 indicates moderate limitation of physical function.
e PROMIS fatigue T-score is defined as normal (≤50), mild (50-55), moderate (55-65), and severe (≥65).
f Patient pain VAS is defined as mild (≤3.4), moderate (3.5-7.4), and severe (≥7.5).
g Patient Global Assessment is defined as mild disease (≤3), moderate (4.1 to < 7), and severe (≥7).
To cite this abstract in AMA style:
Curtis J, Holladay E, Egana A, Lalla A, Su Y, Xie F, Mudano A, Daigle S, McCoy S. Demographic and Clinical Characteristics of Patients Diagnosed With Sjögren’s Disease Using Electronic Health Records and Linked Claims Data From the US Excellence Network in RheumatoloGY (ENRGY) Practice-Based Research Network [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/demographic-and-clinical-characteristics-of-patients-diagnosed-with-sjogrens-disease-using-electronic-health-records-and-linked-claims-data-from-the-us-excellence-network-in-rheumatology-enr/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/demographic-and-clinical-characteristics-of-patients-diagnosed-with-sjogrens-disease-using-electronic-health-records-and-linked-claims-data-from-the-us-excellence-network-in-rheumatology-enr/
