Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tumor necrosis factor-α inhibitors (TNFi) are extensively utilized in the management of rheumatoid arthritis (RA). Therapeutic responses remain highly heterogeneous. Emerging evidence suggests glucagon-like peptide-1 (GLP-1) plays a role in the regulation of inflammatory processes relevant to RA pathophysiology and may influence TNFi efficacy in RA patients1,2. This study reports evidence of GLP-1 therapy contributing to TNFi response, documented by a validated molecular signature response classifier (MSRC) that predicts TNFi treatment outcomes3.
Methods: Data obtained from two independent RA patient cohorts: NETWORK-004, an observational clinical study of 259 samples from 152 unique eligible patients designed to evaluate and validate MSRC3 performance and real-world evidence (RWE) from 34,795 RA patients treated by 1623 physicians across 962 rheumatology clinics in 48 states in the US. GLP-1 treatment records obtained from open claims databases. MSRC scores, ranging from 1 to 25, were evaluated for each patient. A higher MSRC score predicts greater likelihood of inadequate TNFi response. MSRC ≥18.5 is classified as having a very high likelihood of inadequate response.
Results: Study participants demographic information reported in table 1. The analysis of NETWORK-004 demonstrated although no direct linear correlation of body mass index (BMI) and MSRC was observed, a larger proportion of patients with BMI ≥30 (40.9%) compared to BMI < 30 (4.7%) exhibited MSRC score ≥18.5. The difference statistically constitutes a 9-fold increase (Fig 1a). Remarkedly, modeling BMI reduction of 10% to 20% would result in a reduction of MSRC median 8.4% to 17.6%, respectively, indicating that decreasing BMI may lead to decreasing the likelihood of inadequate TNFi response (Fig 1b). Consistent with these findings, examination of the RWE cohort revealed that a larger proportion of patients with BMI ≥30 (24.1%) exhibited very high likelihood of inadequate TNFi response compared to BMI < 30 (4.7%), documented by MSRC score ≥18.5 (Fig 2a). The impact of GLP-1 exposure in the RWE cohort was evaluated. Analysis showed GLP-1 patients achieving BMI < 30 demonstrated a significant reduction in TNFi inadequate response (9.0%) compared to patients with BMI ≥30 (26.5%). The reduction is approximately 194% and equivalent to 2.9-fold decreased likelihood of inadequate TNFi response for GLP-1 patients achieving BMI < 30 (Fig 2b).
Conclusion: In this study, GLP-1-based interventions, traditionally employed in the treatment of metabolic diseases, enhanced TNFi therapeutic efficacy in specific RA patient subgroups. Future randomized clinical trials are warranted to validate the efficacy of GLP-1 receptor agonists and TNF inhibition combined therapies for RA and potentially other autoimmune diseases. In precision medicine, MSRC can serve as a biomarker to identify patients who may benefit from these combinatorial therapeutic regimens.1. Mehdi SF et al. Front Immunol. 2023;14:1148209.2. Bendotti G et al. Pharmacol Res. 2022;182:106320.3. Cohen S et al. Rheumatol Ther. 2021;8(3):1159–1176.
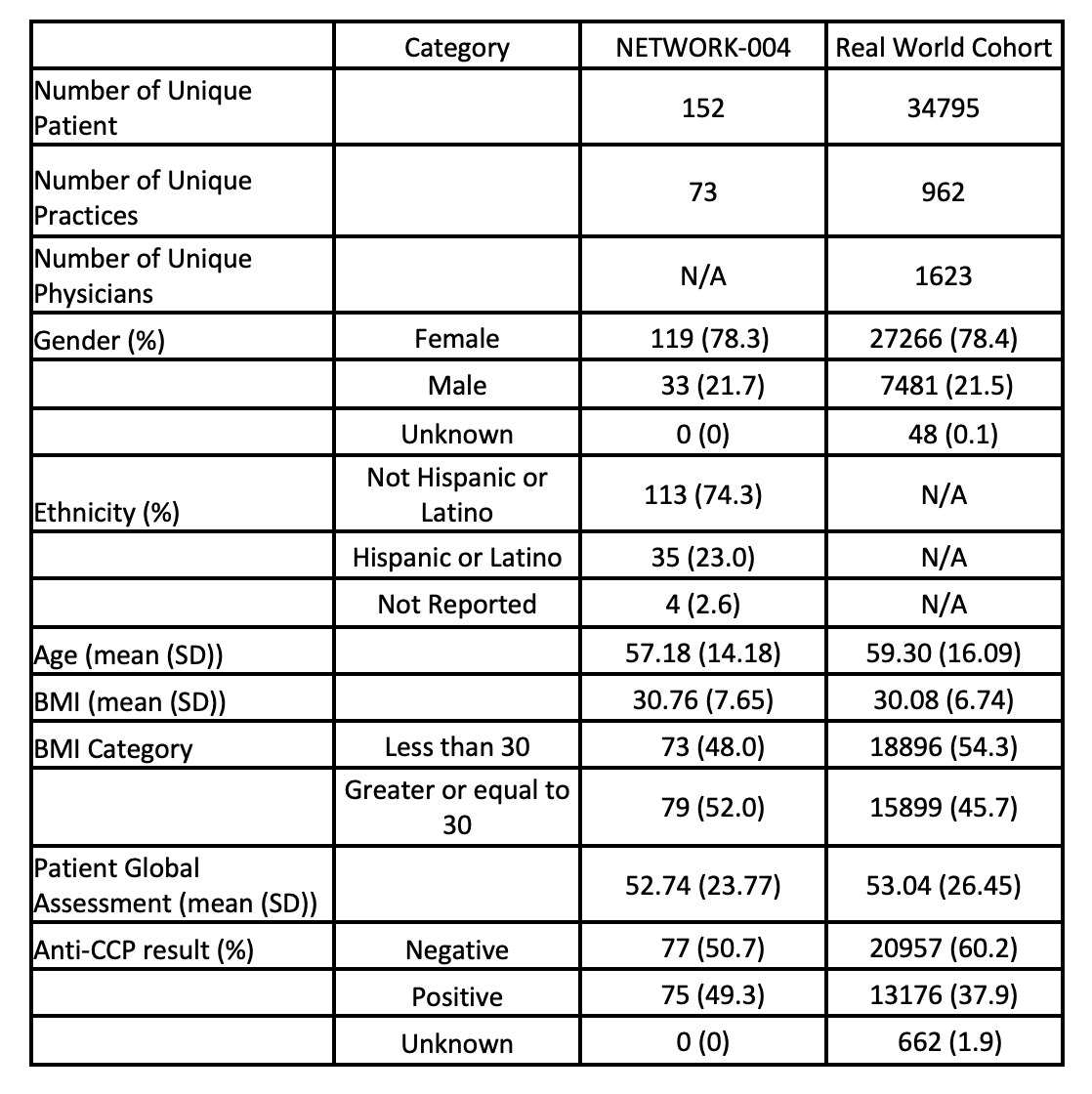 Table 1. Baseline Characteristics for NETWORK-004 study and Real-world cohort
Table 1. Baseline Characteristics for NETWORK-004 study and Real-world cohort
.jpg) Figure 1. BMI Association between inadequate TNFi response predicted by MSRC in NETWORK-004 study. Figure 1a illustrates a 9-fold increase in the proportion of patients with MSRC scores ≥18.5 ( < 5% chance of TNFi response) among those with BMI ≥30 comparing to those with BMI < 30 in the NETWORK-004 cohort, as shown in bar charts with fold change on the y-axis. Figure 1b demonstrates the modeled downward shift in MSRC scores corresponding to hypothetical BMI reductions ranging from 5% to 20% relative to baseline.
Figure 1. BMI Association between inadequate TNFi response predicted by MSRC in NETWORK-004 study. Figure 1a illustrates a 9-fold increase in the proportion of patients with MSRC scores ≥18.5 ( < 5% chance of TNFi response) among those with BMI ≥30 comparing to those with BMI < 30 in the NETWORK-004 cohort, as shown in bar charts with fold change on the y-axis. Figure 1b demonstrates the modeled downward shift in MSRC scores corresponding to hypothetical BMI reductions ranging from 5% to 20% relative to baseline.
.jpg) Figure 2. Real-world evidence from MSRC tested cohort. Figure 1a displays shaded bar charts presenting the proportion of patients with MSRC scores ≥18.5 ( < 5% chance of TNFi response), stratified by BMI group (BMI < 30 vs. BMI ≥30) in the overall cohort, demonstrating a 5.3-fold increase in the higher BMI group. P-values were calculated using chi-square tests. Figure 1b depicts the proportion relative to BMI ≥30 among patients with concurrent or prior GLP-1 treatment, where a reduced 2.9-fold difference is presented.
Figure 2. Real-world evidence from MSRC tested cohort. Figure 1a displays shaded bar charts presenting the proportion of patients with MSRC scores ≥18.5 ( < 5% chance of TNFi response), stratified by BMI group (BMI < 30 vs. BMI ≥30) in the overall cohort, demonstrating a 5.3-fold increase in the higher BMI group. P-values were calculated using chi-square tests. Figure 1b depicts the proportion relative to BMI ≥30 among patients with concurrent or prior GLP-1 treatment, where a reduced 2.9-fold difference is presented.
To cite this abstract in AMA style:
Zhang L, Wong A, Jones A, Guardiano S, Seeto R, Phan R. Molecular Signature Response Classifier Identifies Contribution of GLP-1 Therapy to TNF Inhibitor Response in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/molecular-signature-response-classifier-identifies-contribution-of-glp-1-therapy-to-tnf-inhibitor-response-in-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/molecular-signature-response-classifier-identifies-contribution-of-glp-1-therapy-to-tnf-inhibitor-response-in-rheumatoid-arthritis-patients/
