Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous studies suggest that glucocorticoids are associated with worse survival in patients receiving immune checkpoint inhibitors (ICI). This is an important issue for Rheumatoid Arthritis (RA) patients initiating ICI since they may require glucocorticoids for RA disease control or management of immune related adverse events. Glucocorticoids are also used for cancer palliation, and for this indication dexamethasone is the preferred formulation. We investigated the association between glucocorticoid use and type and overall survival (OS) among patients with pre-existing RA treated with ICI for non-small cell lung cancer (NSCLC).
Methods: We used a curated Medicare claims dataset (2010-2019) that consists of a 100% sample of patients with RA. Inclusion criteria: Age ≥ 66 years; RA preceding NSCLC diagnosis initiating nivolumab, pembrolizumab or atezolizumab (during dates approved only for metastatic/ stage 4 cancer, to homogenize cancer stage). Kaplan Meier (KM) curves and 90-day landmark Cox proportional hazard (PH) models were created to measure OS from first ICI initiation, stratified by glucocorticoid (GC) use in the first 90 days. Adjusted models controlled for age, sex, race, ethnicity, interstitial lung disease, chronic obstructive pulmonary disease, prior radiation, year of ICI initiation, modified Elixhauser score, first- or second-line ICI, concomitant chemotherapy. Steroid use was modeled both as binary (KM curves) and as a continuous variable. Schoenfeld residuals were used to assess the PH assumption. Patients were followed through 12/31/2019, or time of death.
Results: We identified 779 eligible RA patients with mean age 74.7 (SD 7.2) years, 87.8% White, 65% female. Among these, 479 (61%) were treated with GCs in the first 90 day. GC users had similar demographics to the overall cohort (table 1). Among GC users, median dose was 7.9 mg prednisone equivalents/day [IQR 4.1,13.2]. In KM analyses, glucocorticoid use (yes/no) was associated a non-significant trend towards worse overall survival (Figure 1a). In a separate KM curve, dexamethasone use was associated with worse OS compared to non-users (Figure 1b). In adjusted models, higher mean glucocorticoid dose was associated with worse overall survival (aHR 1.08 [95%CI 1.01, 1.16]), but in a sensitivity analysis that excluded dexamethasone-treated patients, the association of glucocorticoid dose with overall survival disappeared [HR 0.91 95%CI [0.83, 1.01].
Conclusion: Glucocorticoids used to treat RA and irAE were not associated with worse survival in NSCLC patients with RA after exclusion of dexamethasone-treatment. Dexamethasone, often given palliatively at high doses to alleviate work of breathing, nausea and/or brain edema in cancer patients, should be excluded (or reason for censoring) from studies assessing the association between glucocorticoid use and survival in patients with RA.
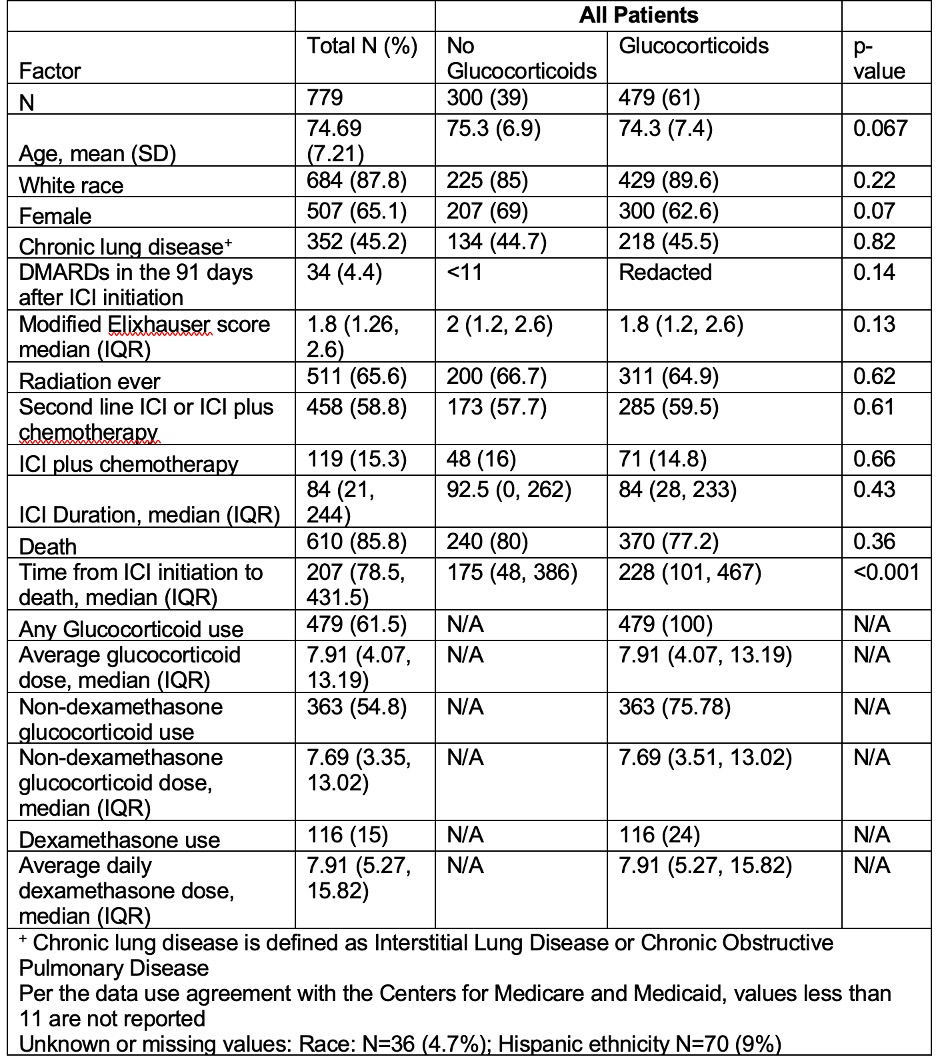 Table 1 Demographic and Clinical Characteristics Rheumatoid Arthritis Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors from Medicare Fee for Service Claims Data.
Table 1 Demographic and Clinical Characteristics Rheumatoid Arthritis Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors from Medicare Fee for Service Claims Data.
.jpg) Figure 1 A) 90 day landmark Kaplan Meier by glucocorticoid use; B) 90 day landmark Kaplan Meier comparing dexamethasone treatment inclusion
Figure 1 A) 90 day landmark Kaplan Meier by glucocorticoid use; B) 90 day landmark Kaplan Meier comparing dexamethasone treatment inclusion
To cite this abstract in AMA style:
Jannat-Khah D, Curtis J, Xie F, Saxena A, Bass A. Are Glucocorticoids Associated with Worse Overall Survival among Rheumatoid Arthritis Patients Treated with Immune Checkpoint Inhibitors? The Confounding Effect of Dexamethasone [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/are-glucocorticoids-associated-with-worse-overall-survival-among-rheumatoid-arthritis-patients-treated-with-immune-checkpoint-inhibitors-the-confounding-effect-of-dexamethasone/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-glucocorticoids-associated-with-worse-overall-survival-among-rheumatoid-arthritis-patients-treated-with-immune-checkpoint-inhibitors-the-confounding-effect-of-dexamethasone/
