Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: IgG4-related disease (IgG4-RD) is a chronic, systemic, immune-mediated disease that is commonly treated with B cell depletion. However, due to potential risks associated with long-term B cell depletion, there is a need for alternative, non-depleting treatment options. Bruton’s tyrosine kinase (BTK) plays a critical role in B cell activation and proliferation, and inhibiting BTK is efficacious in B cell driven diseases, without meaningfully reducing B cell numbers. In this phase 2 proof-of-concept study (NCT04602598), we report the efficacy and safety of zanubrutinib in patients with IgG4-RD affecting the lacrimal and/or salivary glands.
Methods: This open-label study was conducted at a single center in the United States. Eligible adult patients with symptomatic IgG4-RD affecting the submandibular and/or lacrimal glands, with prior inadequate response to glucocorticoids, and meeting the 2019 ACR/EULAR Classification Criteria for IgG4-RD, received oral zanubrutinib 80 mg twice daily for up to 24 weeks. The primary endpoint was the change from baseline in the volume of the submandibular and/or lacrimal glands on 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging (FDG-PET/MRI) at Week 24, as determined by two board-certified radiologists blinded to scan order. Secondary endpoints included the change in metabolic activity on FDG-PET/MRI, laboratory parameters such as IgG4 serum concentration, and patient reported outcomes. The Wilcoxon signed rank exact test was used to evaluate changes on imaging.
Results: Ten patients were enrolled; most were male (70.0%), Asian (60.0%), and with a median age of 58 (range, 38-74) years. Prior treatments included prednisone (10 patients), azathioprine (2 patients), and rituximab (5 patients); for rituximab-treated patients, the mean (standard deviation [SD]) time since last dose was 2.5 years (1.5). No patients were receiving glucocorticoids or a steroid-sparing immunosuppressive treatment at the time of enrollment or during the study. The mean (SD) number of organs ever affected by IgG4-RD was 4.4 (1.4), and all patients had both lacrimal and submandibular gland disease. At Week 24, the mean (SD) percent changes from baseline were -45.4% (17.4) for lacrimal gland volume (p < 0.001) and -30.0% (18.6) for submandibular gland volume (p < 0.001) (Figure 1). In addition, reductions were observed in all measures of metabolic activity, including a mean (SD) change from baseline in total lesion glycolysis of -91.6 grams (98.5; p = 0.05) (Figure 2). Representative FDG-PET/MRI images are shown in Figure 3. The mean (SD) change in the serum IgG4 concentration over 24 weeks was -417.0 mg/dL (410). All patients had at least one treatment-emergent adverse event (TEAE), most of which were mild, and there was one serious TEAE from COVID-19.
Conclusion: Treatment with zanubrutinib alone, without the need for glucocorticoid induction or other background immunosuppression, significantly reduced both lacrimal and submandibular gland volume and metabolic activity on FDG-PET/MRI, as well as reduced serum IgG4 concentrations, with an acceptable safety profile. Given these results, zanubrutinib warrants further investigation as a treatment for IgG4-RD.
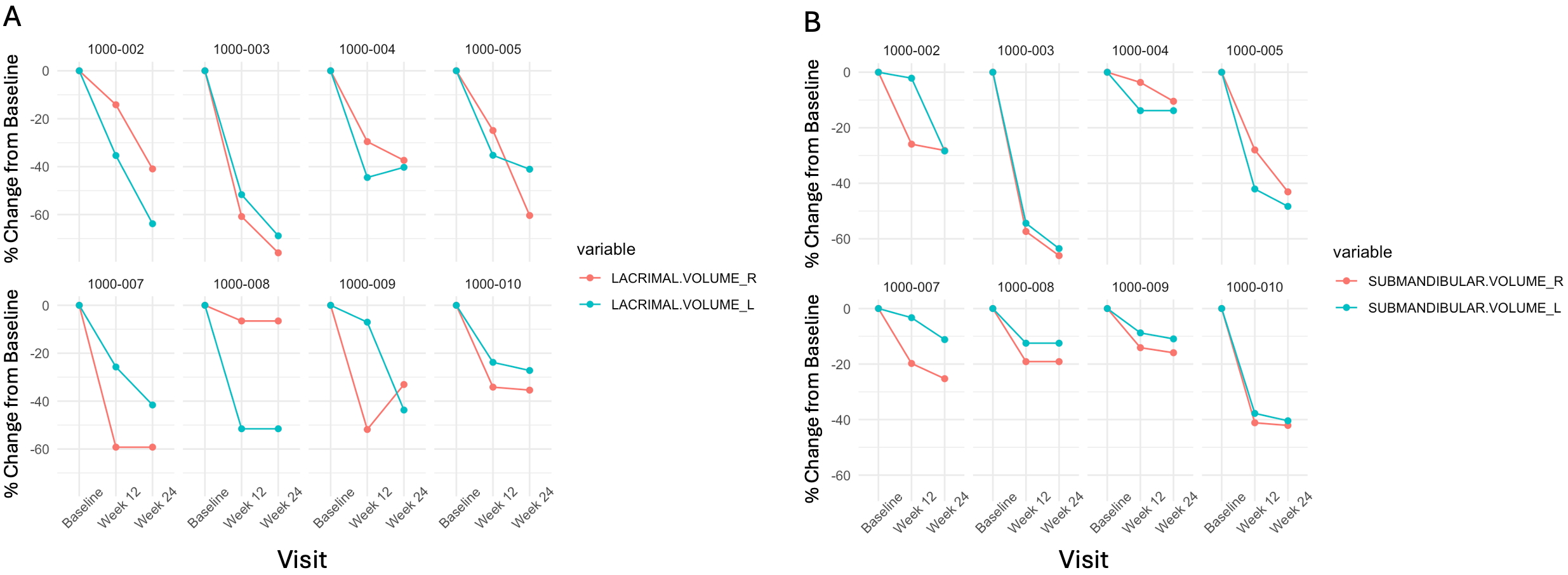 Figure 1. Individual changes in gland volume on FDG-PET/MRI. Panel A shows the percent change from Baseline to Week 12 and Week 24 in lacrimal gland volume for each subject. Panel B shows the percent change from Baseline to Week 12 and Week 24 in submandibular gland volume for each subject. Two subjects who only had Baseline data and terminated early due to adverse events were not included in this analysis. One subject terminated after Week 12 due to worsening IgG4-RD, and the Week 12 imaging data was imputed for Week 24.
Figure 1. Individual changes in gland volume on FDG-PET/MRI. Panel A shows the percent change from Baseline to Week 12 and Week 24 in lacrimal gland volume for each subject. Panel B shows the percent change from Baseline to Week 12 and Week 24 in submandibular gland volume for each subject. Two subjects who only had Baseline data and terminated early due to adverse events were not included in this analysis. One subject terminated after Week 12 due to worsening IgG4-RD, and the Week 12 imaging data was imputed for Week 24.
.jpg) Figure 2. Individual changes in total lesion glycolysis (TLG). TLG is defined as the product of mean standard uptake value (SUVmean) and total metabolic lesion volume (tMLV) of the lacrimal glands, parotid glands, submandibular glands, and regional lymph nodes. Two subjects who only had Baseline data and terminated early due to adverse events were not included in this analysis. One subject terminated after Week 12 due to worsening IgG4-RD, and the Week 12 imaging data was imputed for Week 24.
Figure 2. Individual changes in total lesion glycolysis (TLG). TLG is defined as the product of mean standard uptake value (SUVmean) and total metabolic lesion volume (tMLV) of the lacrimal glands, parotid glands, submandibular glands, and regional lymph nodes. Two subjects who only had Baseline data and terminated early due to adverse events were not included in this analysis. One subject terminated after Week 12 due to worsening IgG4-RD, and the Week 12 imaging data was imputed for Week 24.
.jpg) Figure 3. Change in gland volume and metabolic activity on FDG-PET/MRI. Panel A shows MRI 3D volumetric segmentation and rendering of the lacrimal, parotid, and submandibular glands at Baseline and Week 24. Panel B shows FDG-PET maximum intensity projection (MIP) images in right anterior oblique orientation (SUV scale 0-7), demonstrating hypermetabolism in all glands at Baseline, which significantly improves after treatment.
Figure 3. Change in gland volume and metabolic activity on FDG-PET/MRI. Panel A shows MRI 3D volumetric segmentation and rendering of the lacrimal, parotid, and submandibular glands at Baseline and Week 24. Panel B shows FDG-PET maximum intensity projection (MIP) images in right anterior oblique orientation (SUV scale 0-7), demonstrating hypermetabolism in all glands at Baseline, which significantly improves after treatment.
To cite this abstract in AMA style:
Baker M, Horomanski A, Fairchild R, Liu Y, Diaz Deluna M, Lanz T, Gawde S, Khalighi M, Franc B, Penta M, Pham N, Guja K. A Phase II, Single-Site, Open-Label Study of Zanubrutinib in Patients with IgG4-Related Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-phase-ii-single-site-open-label-study-of-zanubrutinib-in-patients-with-igg4-related-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-ii-single-site-open-label-study-of-zanubrutinib-in-patients-with-igg4-related-disease/
