Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Zasocitinib (TAK-279) is an investigational, highly selective and potent, oral, allosteric tyrosine kinase 2 (TYK2) inhibitor. In a phase 2b trial of moderate-to-severe plaque psoriasis, the primary endpoint (psoriasis area and severity index [PASI] 75 response at Week 12) was met with zasocitinib 5 mg, 15 mg and 30 mg once daily; 33% of patients receiving zasocitinib 30 mg achieved PASI 100. The objective of this post hoc analysis was to evaluate the influence of baseline characteristics on zasocitinib efficacy in patients with plaque psoriasis.
Methods: This phase 2b, randomized, multicenter, double-blind, placebo (PBO)-controlled, multiple-dose study (NCT04999839) included post hoc analyses of Week 12 PASI 75/90/100 responses, Physician Global Assessment (PGA) scores (clear [0] or almost clear [1]) and Dermatology Life Quality Index (DLQI) scores stratified by weight, sex, age, race, disease duration, prior biologics use and baseline PASI. Treatment differences (zasocitinib versus PBO) for all subgroups except prior biologic treatment were calculated using the Mantel–Haenszel method; 95% confidence intervals and p values were calculated using the Wald method and Cochran–Mantel–Haenszel test (stratified for prior biologic treatment), respectively. Treatment difference estimates were unadjusted for the subgroup analysis by prior biologic treatment; p values were calculated using a chi-square test. For DLQI, a mixed model for repeated measures was used to calculate the least-squares mean, with treatment, visit, treatment-by-visit interaction and prior biologic treatment (not included for the subgroup analysis by prior biologic treatment) as fixed effects and baseline score as the covariate.
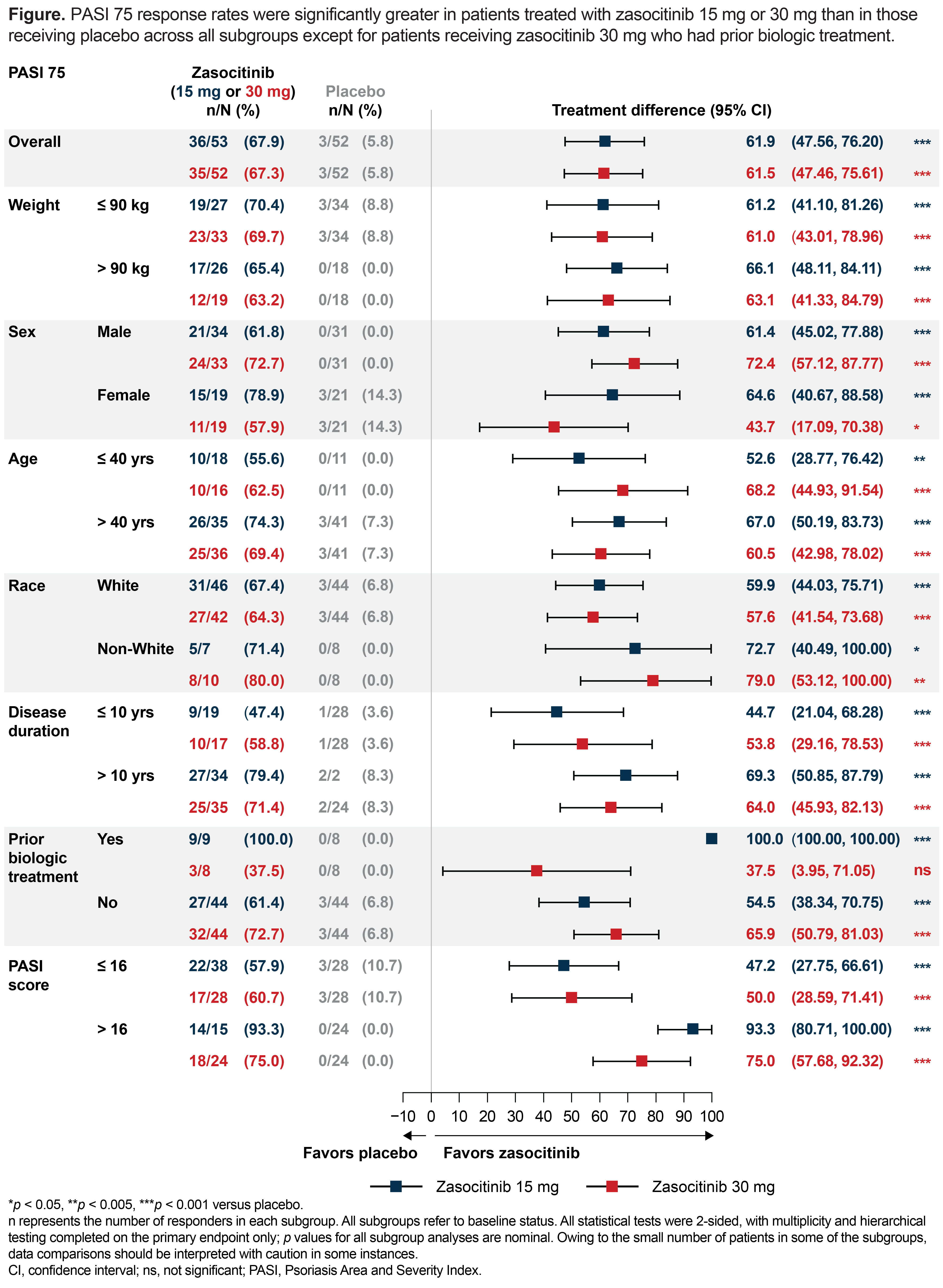
Results: Overall, 259 patients were included in this study. PASI 75 response rates with zasocitinib were greater than PBO regardless of weight (≤ 90kg: PBO, 8.8%; 15 mg, 70.4%; 30 mg, 69.7%; > 90kg: PBO, 0%; 15 mg, 65.4%; 30 mg, 63.2%, each p < 0.001), sex (male: PBO, 0%; 15 mg, 61.8%; 30 mg, 72.7%, each p < 0.001; female: PBO, 14.3%; 15 mg, 78.9% [p < 0.001]; 30 mg, 57.9% [p < 0.005]), age (≤ 40 years: PBO, 0%; 15 mg, 55.6% [p < 0.01]; 30 mg, 62.5% [p < 0.001]; > 40 years: PBO, 7.3%; 15 mg, 74.3%; 30 mg, 69.4%, each p < 0.001), race (White: PBO, 6.8%; 15 mg, 67.4%; 30 mg, 64.3%, each p < 0.001; non-White: PBO, 0%; 15 mg, 71.4% [p < 0.01]; 30 mg, 80.0% [p < 0.005], disease duration (≤ 10 years: PBO, 3.6%; 15 mg, 47.4%; 30 mg, 58.8%; > 10 years: PBO, 8.3%; 15 mg, 79.4%; 30 mg, 71.4%, each p < 0.001), prior biologics use (yes: PBO, 0%; 15 mg, 100% [p < 0.001]; 30 mg, 37.5% [p = 0.055, not significant]; no: PBO, 6.8%; 15 mg, 61.4%; 30 mg, 72.7%, each p < 0.001) and baseline PASI (≤ 16: PBO, 10.7%; 15 mg, 57.9%; 30 mg, 60.7%; > 16: PBO, 0%; 15 mg, 93.3%; 30 mg, 75.0%, each p < 0.001; Figure). PASI 90, PASI 100, PGA 0/1 and DLQI generally improved with zasocitinib treatment versus PBO in almost all subgroups.
Conclusion: Treatment with zasocitinib 15 mg or 30 mg led to greater response rates at Week 12 versus PBO in patients with moderate-to-severe plaque psoriasis, for nearly all baseline characteristics assessed. Phase 3 trials (NCT06088043 and NCT06108544) are ongoing to investigate the efficacy and safety of zasocitinib in larger patient groups.
To cite this abstract in AMA style:
Elbuluk N, Gooderham M, Blau J, Zhang W, Mark E, Winkelman W, Lebwohl M. Zasocitinib (TAK-279), an Investigational, Oral, Allosteric, Selective TYK2 Inhibitor, in Moderate-to-Severe Plaque Psoriasis: Efficacy Analysis by Baseline Characteristics from a Randomized Phase 2b Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/zasocitinib-tak-279-an-investigational-oral-allosteric-selective-tyk2-inhibitor-in-moderate-to-severe-plaque-psoriasis-efficacy-analysis-by-baseline-characteristics-from-a-randomized-phase-2b/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zasocitinib-tak-279-an-investigational-oral-allosteric-selective-tyk2-inhibitor-in-moderate-to-severe-plaque-psoriasis-efficacy-analysis-by-baseline-characteristics-from-a-randomized-phase-2b/