Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite evidence-based recommendations, allopurinol dose escalation to goal serum urate (SU) is frequently suboptimal. The EasyAllo tool was developed to facilitate pre-planned allopurinol dose escalation by predicting the allopurinol dose needed to achieve SU < 6 mg/dL >80% of the time and promote easier dose titration (Wright et al. Br J Clin Pharmacol 2024). The purpose of this study was to externally validate EasyAllo among trial participants who achieved goal SU using protocolized dose escalation.
Methods: We included participants in the STOP Gout trial who were randomized to the allopurinol arm, and limited our analyses to those without tophi at baseline who achieved goal SU < 6 mg/dL and completed week 48 of the study to ensure adequate dose escalation and SU capture. All participants fulfilled the 2015 ACR-EULAR gout classification criteria. The trial used protocolized allopurinol dose escalation if SU was ≥6 mg/dL through week 30 to a maximum of 800 mg daily. Two versions of EasyAllo exist: EasyAllo2 based on weight and creatinine clearance and EasyAllo1 which also incorporates baseline SU. We used EasyAllo2 in the primary analysis to allow for inclusion of participants taking allopurinol at enrollment whose baseline SU reflected active treatment, and secondarily explored EasyAllo1 among those not already taking allopurinol. In primary analysis, we determined the frequency with which the study dose required to achieve SU goal < 6 mg/dL between weeks 36 and 48 was at the EasyAllo2 predicted dose or lower. As a secondary aim, we assessed univariate associations between baseline characteristics and being at the EasyAllo predicted dose or lower. We also evaluated whether participants who study dose was the EasyAllo2 predicted dose at the time of first achieving SU < 6 mg/dL demonstrated superior long-term maintenance of SU < 6 mg/dL compared to participants whose study dose was less than the predicted dose.
Results: A total of 291 participants met inclusion criteria for the primary analysis (Table 1). Approximately 77% (n = 224) of participants who achieved SU goal < 6 mg/dL between weeks 36 and 48 were on the EasyAllo2 predicted dose or less. Younger age, lower kidney function, and higher SU were associated with requiring allopurinol doses higher than predicted by EasyAllo2 (Table 3). In secondary analysis using EasyAllo1, which includes baseline SU for prediction, younger age, higher baseline weight, and higher SU were associated with allopurinol doses higher than predicted. Considering all participants who ever achieved SU < 6 mg/dL, half (n=207, 51%) subsequently had SU that was not at goal (≥6 mg/dL). This occurred more frequently among participants on a study dose below the EasyAllo2 predicted dose relative to participants on a study dose at or above the EasyAllo2 dose prediction, though, this difference did not achieve statistical significance (55% v 45%, p = 0.06).
Conclusion: Among participants who achieved SU < 6.0 mg/dL through protocolized allopurinol dose escalation, nearly 3 out of 4 would likely have achieved SU goal at the EasyAllo2 predicted dose. These findings suggest that EasyAllo2 would perform well in this population with a small proportion requiring additional dose escalation.
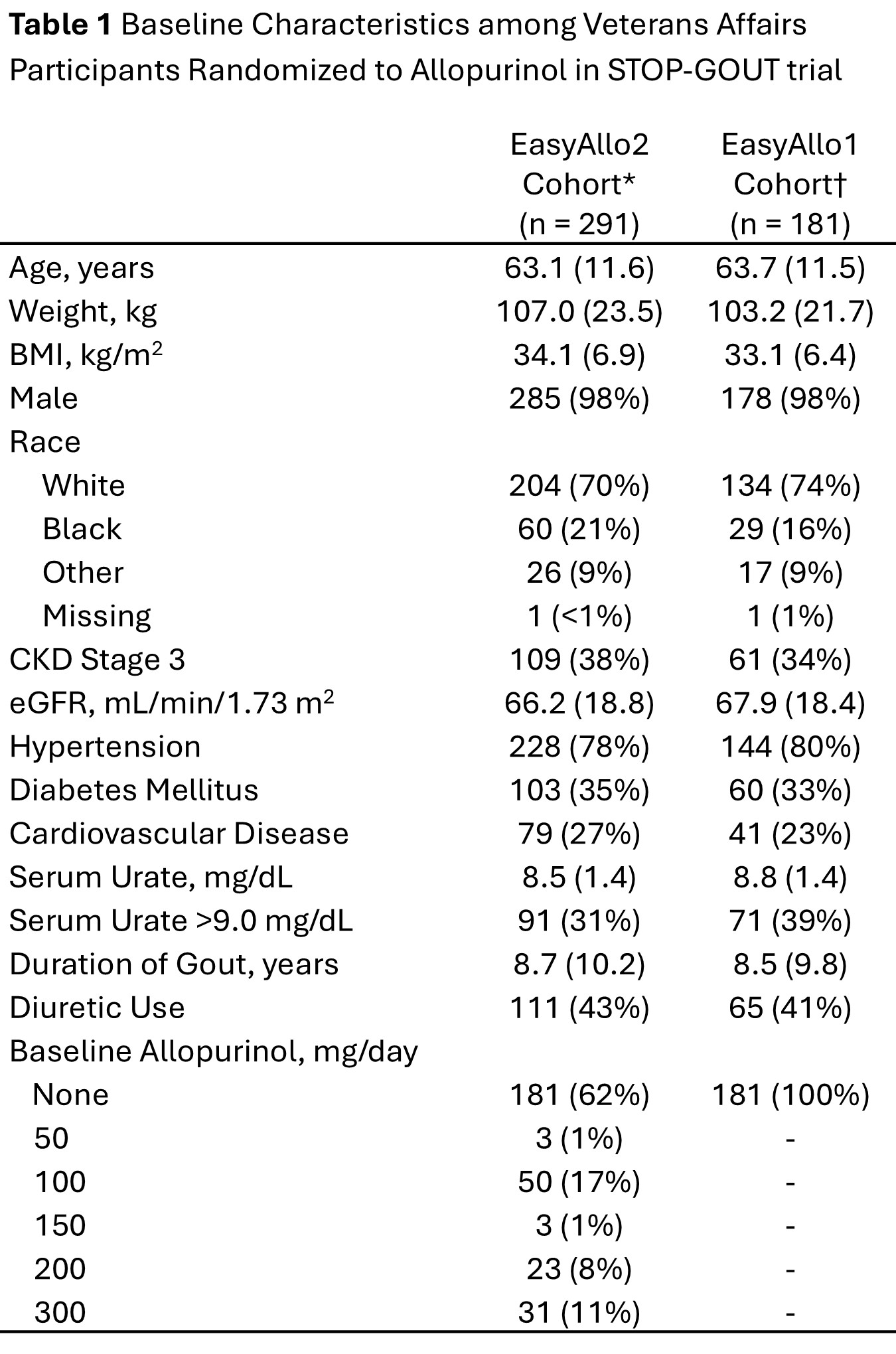 All values are mean (sd) or n (%). *The EasyAllo2 cohort is limited to participants without tophi at baseline who achieved goal SU < 6 mg/dL and completed week 48 of the study to ensure adequate dose escalation and SU capture. †The EasyAllo1 cohort is further limited to participants not on baseline allopurinol to ensure baseline SU values do not reflect active treatment. Abbreviations: body mass index (BMI); chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR)
All values are mean (sd) or n (%). *The EasyAllo2 cohort is limited to participants without tophi at baseline who achieved goal SU < 6 mg/dL and completed week 48 of the study to ensure adequate dose escalation and SU capture. †The EasyAllo1 cohort is further limited to participants not on baseline allopurinol to ensure baseline SU values do not reflect active treatment. Abbreviations: body mass index (BMI); chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR)
.jpg) All doses represent mg per day of allopurinol. A total of 291 participants in the allopurinol group without baseline tophi met goal SU < 6 mg/dL between weeks 36 and 48. Green represents participants in whom the final trial dose at the time of achieving SU goal < 6 mg/dL was equal to (n = 85, 29%) or less than the EasyAllo2 predicted dose (n = 139, 48%). Orange represents participants whose final study dose was more than the EasyAllo2 predicted dose (n = 67, 23%). A total of 79% (n = 231) participants were on a study dose within 100 of the EasyAllo2 dose prediction. The weighted kappa, a measure of agreement where doses closer together are weighted more, was 0.27 using quadratic weighting. *EasyAllo2 dose predictions were calculated using participants baseline weight and creatinine clearance. Abbreviations: serum urate (SU)
All doses represent mg per day of allopurinol. A total of 291 participants in the allopurinol group without baseline tophi met goal SU < 6 mg/dL between weeks 36 and 48. Green represents participants in whom the final trial dose at the time of achieving SU goal < 6 mg/dL was equal to (n = 85, 29%) or less than the EasyAllo2 predicted dose (n = 139, 48%). Orange represents participants whose final study dose was more than the EasyAllo2 predicted dose (n = 67, 23%). A total of 79% (n = 231) participants were on a study dose within 100 of the EasyAllo2 dose prediction. The weighted kappa, a measure of agreement where doses closer together are weighted more, was 0.27 using quadratic weighting. *EasyAllo2 dose predictions were calculated using participants baseline weight and creatinine clearance. Abbreviations: serum urate (SU)
.jpg) All values are mean (sd) or n (%) and p-values represent testing for association between baseline participant characteristics and SU goal attainment on the predicted allopurinol dose or lower at week 30 of the study. P-values represent t-test and chi-square test for continuous and categorical variables respectively. EasyAllo2 dose predictions were calculated using participants baseline weight and creatinine clearance and EasyAllo1 calculations also use baseline SU. Abbreviations: serum urate (SU); body mass index (BMI); chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR)
All values are mean (sd) or n (%) and p-values represent testing for association between baseline participant characteristics and SU goal attainment on the predicted allopurinol dose or lower at week 30 of the study. P-values represent t-test and chi-square test for continuous and categorical variables respectively. EasyAllo2 dose predictions were calculated using participants baseline weight and creatinine clearance and EasyAllo1 calculations also use baseline SU. Abbreviations: serum urate (SU); body mass index (BMI); chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR)
To cite this abstract in AMA style:
Coburn B, Wright D, Newcomb J, Brophy M, Davis-Karim A, Ferguson R, Pillinger M, Neogi T, Palevsky P, England B, O'Dell J, Stamp L, Mikuls T, Baker J. Validation of an Allopurinol Dose Prediction Tool to Achieve Goal Serum Urate Among Patients with Gout [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validation-of-an-allopurinol-dose-prediction-tool-to-achieve-goal-serum-urate-among-patients-with-gout/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-an-allopurinol-dose-prediction-tool-to-achieve-goal-serum-urate-among-patients-with-gout/
