Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) has been recommended [1-3] as a steroid-sparing agent for immune checkpoint inhibitor-inflammatory arthritis (ICI-IA), preferred due to its low immunosuppressive effect. However, there is little published data on its efficacy for ICI-IA; to date, the largest study is a case series of 11 patients [4]. This study’s aim was to further assess the safety and effectiveness of HCQ for treatment of rheumatic immune-related adverse events (irAEs), including ICI-IA.
Methods: This retrospective observational study included patients at a large academic medical center who were treated with HCQ for rheumatic irAEs by rheumatologists from 2017 to 2025. Outcomes were ascertained by manual chart review. The primary outcome was arthritis control defined as grade 1 arthritis with HCQ monotherapy with grade 1 arthritis defined as at most mild pain not impacting activities of daily living [3]. HCQ and prednisone dose were recorded at the time of HCQ discontinuation or, if HCQ therapy was ongoing, at the time of data collection. Subgroup analyses were also performed.
Results: The study included 40 patients with mean age at HCQ initiation of 69 years (SD 10 years) (Table 1). 25 (62.5%) patients were on anti-PD-1/PD-L1 monotherapy and 9 (22.5%) were on dual therapy with both anti-PD-1/PD-L1 and anti-CTLA-4 ICIs. Mean follow-up time was 29 months (SD 24 months) with 32 patients living at the time of chart review, 8 (25%) of whom remained on HCQ therapy. Among the 27 patients who discontinued HCQ (excluding those 5 patients who were on HCQ at time of death), 13 (48.1%) discontinued due to medication intolerance or an adverse event, 5 (18.5%) discontinued due to resolution of arthritis, and 5 (18.5%) discontinued due to inefficacy. 25 (62.5%) patients were treated with HCQ monotherapy only while 15 (37.5%) were treated with additional disease-modifying antirheumatic drug(s) (DMARD) (Table 2). 17 (42.5%) patients achieved grade 1 arthritis, noting that this includes 4 who required ongoing prednisone therapy with mean dose of 5.5 mg/day (SD 3.3 mg/day). 13 (32.5%) patients achieved grade 1 arthritis on HCQ monotherapy without concomitant prednisone dosing > 5 mg daily or subsequent escalation of DMARD therapy. Among these 13 patients, only 5 remained on ICI therapy following addition of HCQ. Seronegative patients (RF/CCP-, ANA-) had slightly higher rates of response than seropositive patients (Table 3).
Conclusion: In this largest cohort to date observing patients treated with HCQ for rheumatic irAEs, we found a high rate of HCQ intolerance, in line with prior studies in SLE and RA [5, 6], noting that symptoms were often nonspecific and attributable to other treatments. While there was moderate response to HCQ therapy, many patients required escalation of DMARD therapy to achieve disease control. Most patients who achieved grade 1 arthritis after HCQ initiation had not resumed ICI therapy. These findings suggest that HCQ may be effective for a subset of patients, particularly those with mild arthritis or in cases where continuation of ICI therapy is not planned. References:[1] PMID: 32168068[2] PMID: 36270461[3] PMID: 34724392 [4] PMID: 30701346[5] PMID: 10229401[6] PMID: 1485814
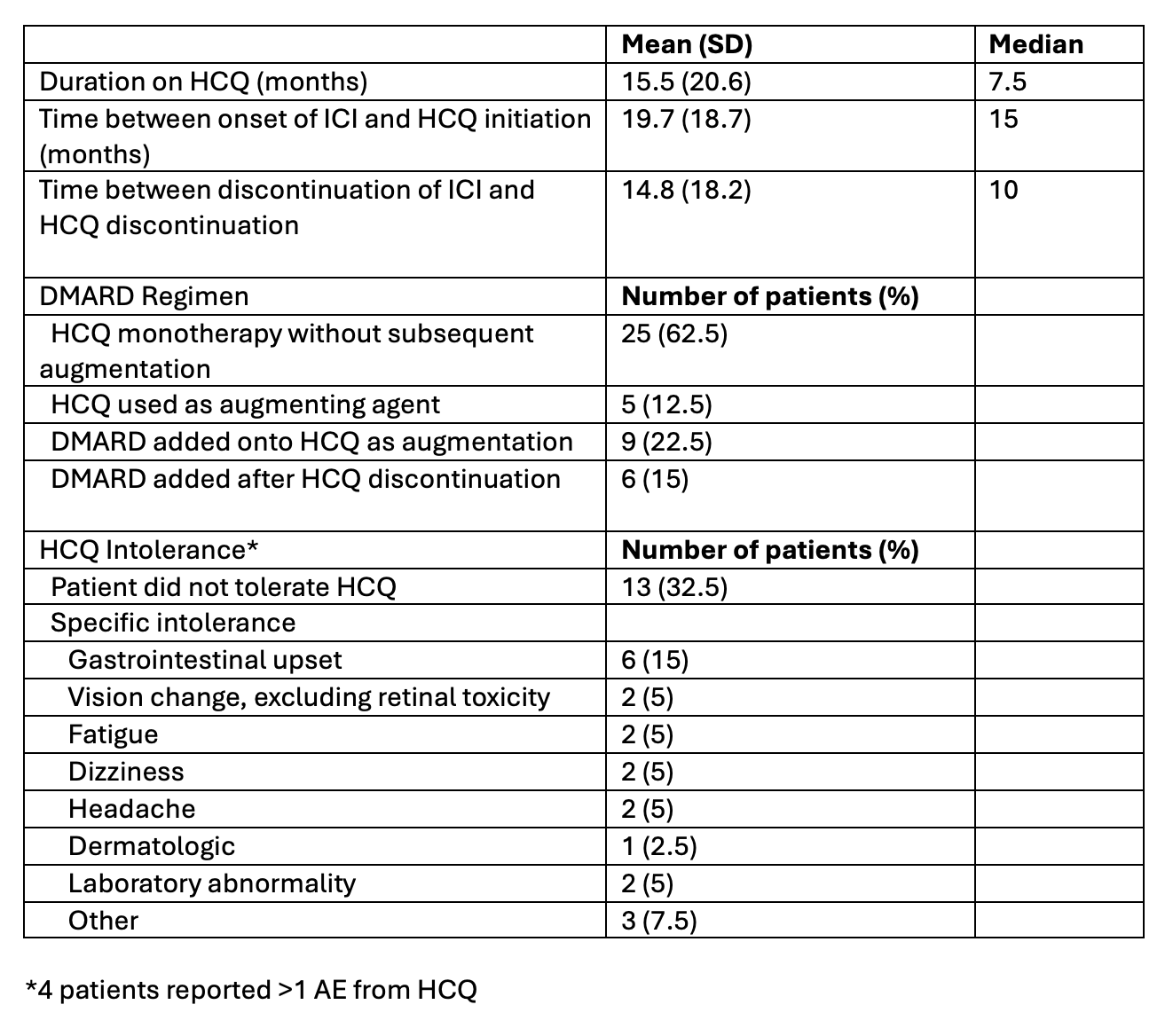 Table 1: Patient characteristics
Table 1: Patient characteristics
.jpg) Table 2: Hydroxychloroquine utilization
Table 2: Hydroxychloroquine utilization
.jpg) Table 3: Subgroup analyses by ICI, serostatus, HCQ regimen, and arthritis type
Table 3: Subgroup analyses by ICI, serostatus, HCQ regimen, and arthritis type
To cite this abstract in AMA style:
Lee G, Challener G, Yinh J, Sparks J, Reynolds K, Lawrence D, Mooradian M, Sullivan R, Choi H, Kohler M. Safety and Effectiveness of Hydroxychloroquine in the Treatment of Rheumatic Immune-Related Adverse Events [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-and-effectiveness-of-hydroxychloroquine-in-the-treatment-of-rheumatic-immune-related-adverse-events/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-effectiveness-of-hydroxychloroquine-in-the-treatment-of-rheumatic-immune-related-adverse-events/
