Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune-related adverse events (irAEs) are a significant concern for patients receiving immune checkpoint inhibitors (ICIs), particularly those with pre-existing rheumatoid arthritis (RA). Managing ICIs in RA patients is complex due to potential immune activation leading to disease flares or new autoimmune manifestations. Although RA patients were often excluded from ICI trials, their increasing representation among cancer patients highlights the need for a better understanding of the safety and toxicity of ICIs in this population.
Methods: We conducted a propensity score-matched cohort study using the TriNetX Analytics Network database, comprising anonymized data from over 140 collaborating healthcare institutions worldwide. We compared the incidence and risk of irAEs in cancer patients with and without RA. Adult patients (age ≥18) receiving ICIs were included. The primary outcome was composite irAEs within one year of ICI initiation, while secondary outcomes were individual irAEs and all-cause mortality. Matching criteria included demographics (age, sex), metastatic disease, chemotherapy and radiation history, underlying comorbidities, ECOG performance status, and cardiovascular medication use. Risk of irAEs was assessed using Cox proportional hazards models, with hazard ratios (HR) and 95% confidence intervals (CI). P-values < 0.05 were considered significant.
Results: We included 969 RA patients and 969 non-RA patients after matching. The mean age was similar between groups (66.2 ± 10.9 years for RA vs. 66.7 ± 12.0 years for non-RA). The proportion of male patients was also comparable (43.6% in RA vs. 44.6% in non-RA). The incidence of irAEs in the RA group was not significantly different from the non-RA group (HR 1.13, 95% CI 0.99–1.29, p=0.072). Notably, the RA group exhibited a significantly lower all-cause mortality (HR 0.84, 95% CI 0.71–1.00, p=0.049). Specific irAEs with increased risk in the RA group included adrenal insufficiency (HR 1.60, p=0.033) and other rheumatic irAEs (HR 3.44, p=0.001). No significant differences were observed in the incidence of colitis, hepatitis, pancreatitis, pneumonitis, myocarditis/pericarditis, or other irAEs.
Conclusion: In this analysis, RA patients receiving ICIs demonstrated no significant difference in the incidence of composite irAEs and a lower risk of all-cause mortality compared to non-RA patients. The findings underscore the importance of close monitoring and multidisciplinary care for RA patients undergoing ICI therapy to mitigate immune toxicity while optimizing cancer outcomes.
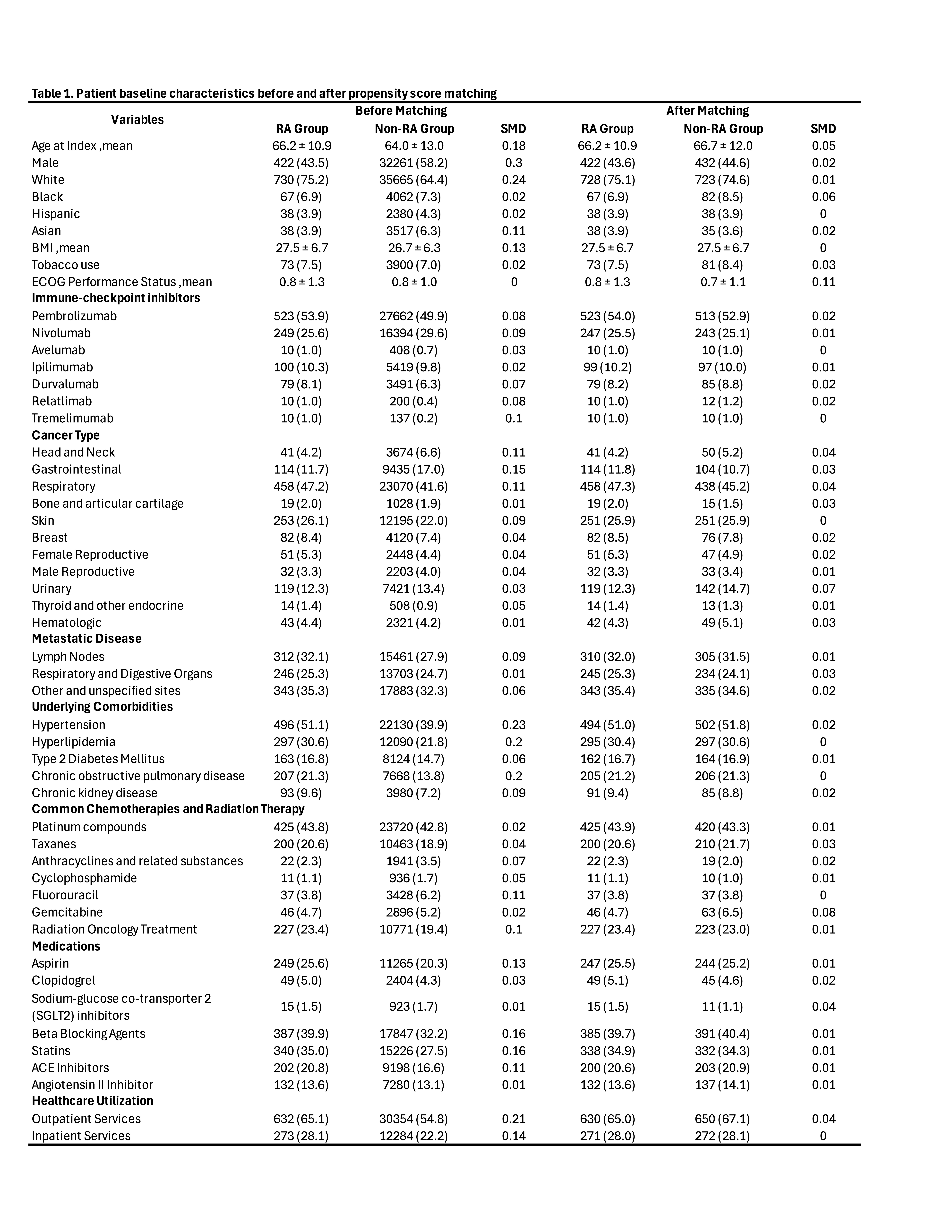 Table 1. Patient baseline characteristics before and after propensity score matching
Table 1. Patient baseline characteristics before and after propensity score matching
.jpg) Table 2. Table 1. Mortality and risk of irAEs among patients with RA vs non-RA group
Table 2. Table 1. Mortality and risk of irAEs among patients with RA vs non-RA group
To cite this abstract in AMA style:
Ibis B, Bahar F, Lee Y, Chang K, Chang Y, Chiang C. Safety Profile of ICIs in Patients with Rheumatoid Arthritis: Results from a Propensity Score-Matched Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-profile-of-icis-in-patients-with-rheumatoid-arthritis-results-from-a-propensity-score-matched-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profile-of-icis-in-patients-with-rheumatoid-arthritis-results-from-a-propensity-score-matched-cohort-study/
