Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Although achieving target serum urate (SU) and reducing cardiovascular events are cornerstones of gout management, little is known about contemporary treatment patterns across racial and ethnic groups. We compared long-term exposure to urate-lowering therapy (ULT), acute-attack medications, procedures, SU, and major adverse cardiovascular events (MACE) between non-Hispanic White (NHW) and racial/ethnic minority (MIN) adults in routine U.S. practice.
Methods: We conducted a retrospective cohort study using TriNetX Collaborative Network. Adults ≥ 18 years with an incident gout diagnosis (ICD-10 M10) were classified as NHW or MIN (Black, Hispanic, Asian)- based on electronic health records. One-to-one propensity matching balanced age, sex, nine comorbidities, cardiometabolic drugs and BMI, yielding 359 020 patients per cohort. Outcomes captured 1–10 y post-index were initiation of allopurinol, febuxostat, pegloticase, colchicine, oral corticosteroids, NSAIDs, IL-1 inhibitors, arthrocentesis, most-recent SU, and composite MACE (myocardial infarction, heart failure, stroke). Risk ratios (RR, 95 % CI) and Kaplan–Meier log-rank tests (α = 0.05) compared groups.
Results: Baseline characteristics were well balanced. During the follow-up period, NHW patients were modestly more likely to start allopurinol than MIN patients (29.8 % vs 28.1 %; RR 1.06, 1.05–1.07), Conversely, febuxostat initiation was nearly twice as common among MIN patients (3.8 % vs 2.1 %; RR 0.56 [0.55–0.58]; p < 0.001). Pegloticase use remained rare at 0.05 % in both cohorts (RR 1.13 [0.92–1.38]; p = 0.25).Acute-therapy patterns differed: colchicine was prescribed to 20.1 % of MIN versus 14.3 % of NHW patients (RR 1.40 [1.38–1.42]; p < 0.001); NSAIDs to 32.4 % versus 30.5 % (RR 1.07 [1.06–1.08]; p < 0.001); and systemic corticosteroids to 46.0 % versus 45.5 % (RR 1.01 [1.00–1.02]; p = 0.001). IL-1 inhibitors, although infrequent, were used in 0.14 % of MIN versus 0.08 % of NHW patients (RR 1.69 [1.43–2.03]; p < 0.001). Arthrocentesis or joint injection occurred in 7.3 % of MIN versus 8.6 % of NHW patients (RR 0.85 [0.83–0.87]; p < 0.001).Biochemically, mean serum urate remained higher in MIN than NHW patients (6.71 ± 2.32 vs 6.39 ± 2.08 mg/dL; p < 0.001). Clinically, major adverse cardiovascular events occurred in 16.8 % of MIN versus 15.5 % of NHW patients (RR 1.08 [1.06–1.10]; p < 0.001), and Kaplan–Meier curves showed earlier event accrual in MIN patients (log-rank p < 0.001).
Conclusion: Despite similar comorbidity profiles, minority adults with gout were less likely to initiate guideline-preferred allopurinol yet more likely to receive febuxostat and multiple acute agents, had higher residual SU, and experienced greater cardiovascular morbidity than non-Hispanic-White adults. These findings highlight treatment and outcome inequities and call for targeted dose-titration protocols, SU monitoring, and cardiovascular-risk mitigation strategies to improve gout care in underserved populations.
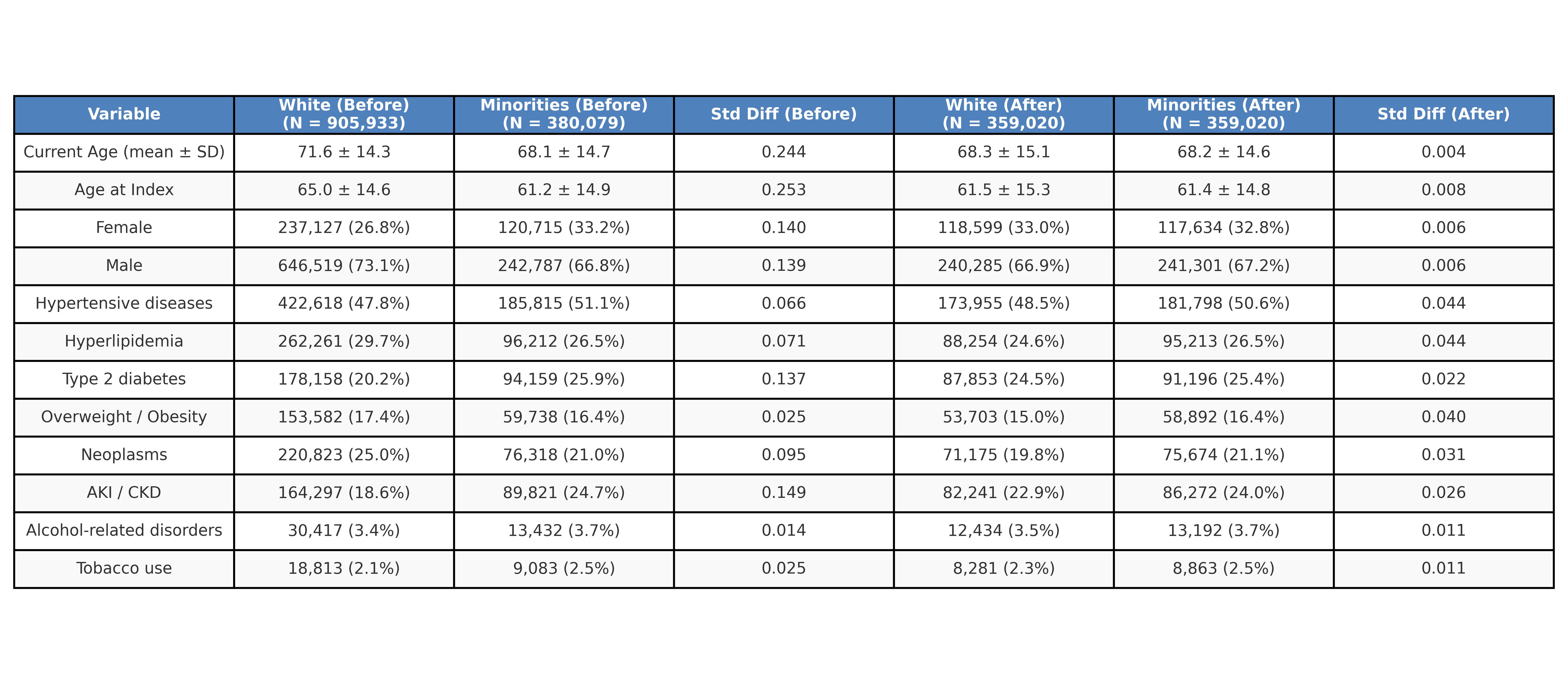 Table 1: Propensitiy score matching-Demographics and baseline characteristics
Table 1: Propensitiy score matching-Demographics and baseline characteristics
.jpg) Propensity score matching- Medications
Propensity score matching- Medications
To cite this abstract in AMA style:
Hamilton M, Lam J, Otabor E, Alomari L, Barnett M, Lau A, Tan I. Racial Differences in Real-World Use of Urate-Lowering and Adjunctive Therapies for Gout: A 10-Year Propensity-Matched Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/racial-differences-in-real-world-use-of-urate-lowering-and-adjunctive-therapies-for-gout-a-10-year-propensity-matched-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-differences-in-real-world-use-of-urate-lowering-and-adjunctive-therapies-for-gout-a-10-year-propensity-matched-cohort-study/

.jpg)