Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Guidelines recommend limiting chronic prednisone use ( >5 mg/d for >3-6 mos.) given higher risks of fractures, infections, and damage. Yet, patients struggle to stop/reduce prednisone due to its perceived rapid benefits despite long-term harms. Shared decision-making (SDM) tools can help clinicians facilitate difficult conversations and support patients in such decisions. There are no feasible SDM tools for chronic prednisone use. Thus, we developed and piloted the PRED-SAFE SDM tool to resolve decisional conflict around chronic prednisone use.
Methods: PRED-SAFE was created using a collaborative process involving a multidisciplinary panel of patients, clinicians, implementation scientists, designers, and literacy experts across 4 institutions (n=23; Fig 1). Initial prototypes were informed by AHRQ low literacy and SDM tool development guidelines, key themes about prednisone in literature and based on interviews with patients, clinicians, experts (n=23). The multidisciplinary panel reviewed the first prototype and guided an iterative process to revise subsequent prototypes until a final version was endorsed by all 23 advisors. The final PRED-SAFE SDM tool was implemented in 4 clinics during 30 visits to examine usability and feasibility. Usability was measured by patient-reported understanding of cumulative harms vs. limited benefits with chronic prednisone use and resolution of decisional conflict using SURE scale (Score >3 shows no residual decisional conflicts) post PRED-SAFE. Using a 1-9 Likert scale (9 = best) to measure users’ likelihood to recommend PRED-SAFE, we estimated Net Promoter Score (NPS = %promoters (extremely/very likely) – % detractors (extremely/very unlikely)). Feasibility of tool use in clinics was assessed using clinician-reported time to review and feasibility & likelihood to use on a 1-9 Likert scale (9=best).
Results: PRED-SAFE SDM laminated tool organized data in pictograms across 6 domains: a) bone fracture, b) infections, c) organ damage, d) cardiovascular disease (CVD), e) flares, f) taper (Fig 2A-D). During visits, a patient & clinician first reviewed increased chances of fracture with chronic prednisone vs. dose ≤5mg/d, followed by higher chance of infections, organ damage, and CVD with chronic prednisone. Limited benefits of chronic prednisone included slight decline in flares with >5mg/d. Finally, steps to taper prednisone were reviewed (Fig 2A-D). PRED-SAFE was reviewed in 4 clinics by 6 clinicians across 30 visits (Table 1). All patients reported improved knowledge of prednisone’s benefits vs. harms; 93% reported no residual decisional conflict post-review (Table 1). PRED-SAFE garnered high usability scores from clinicians (NPS = 100%, mean = 8.9) & patients (NPS = 73%, mean = 8). Mean time to review was 6 mins; 33% of discussions were led by PharmDs/trainees. 90% of clinicians reported using PRED-SAFE was extremely/very feasible and easy for routine use (mean = 8.8; 8.7) (Table 1).
Conclusion: PRED-SAFE is a patient-centered, evidence-based, feasible, easy to use shared decision-making tool that reduces decisional conflict and encourages patient participation in decision-making around chronic prednisone use.
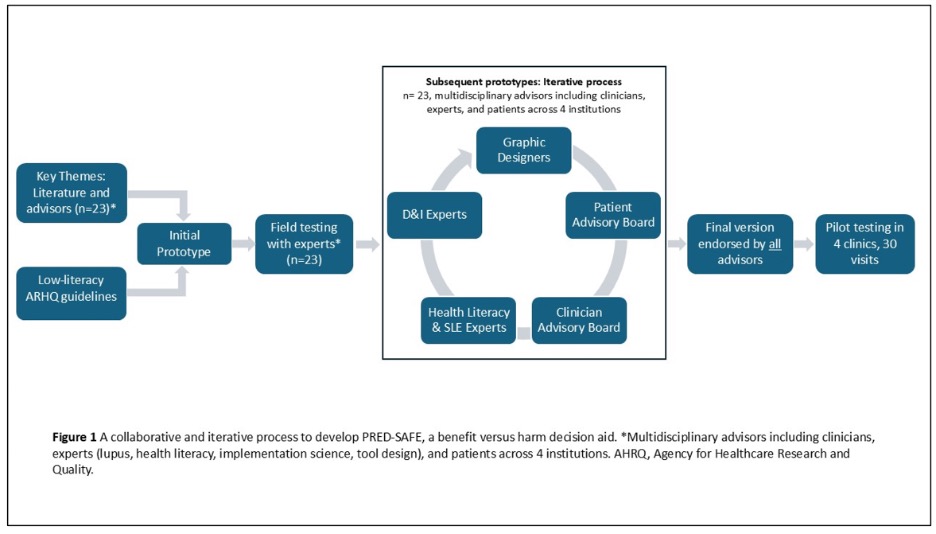 Figure 1. A Collaborative and Iterative Process to Develop PRED-SAFE, a Benefits vs. Harms Shared Decision-Making Tool Around Chronic Prednisone Use
Figure 1. A Collaborative and Iterative Process to Develop PRED-SAFE, a Benefits vs. Harms Shared Decision-Making Tool Around Chronic Prednisone Use
.jpg) Figure 2. PRED-SAFE SDM Tool Domains Showing Harms (Panel A. Bone Fracture; Panel B. Infections) vs. Benefits (Panel C. Flares) of chronic prednisone, & patient information with a taper guide (Panel D)
Figure 2. PRED-SAFE SDM Tool Domains Showing Harms (Panel A. Bone Fracture; Panel B. Infections) vs. Benefits (Panel C. Flares) of chronic prednisone, & patient information with a taper guide (Panel D)
.jpg) Table 1 Pilot testing of PRED-SAFE, a shared decision-making tool for long-term prednisone use, n=30
Table 1 Pilot testing of PRED-SAFE, a shared decision-making tool for long-term prednisone use, n=30
To cite this abstract in AMA style:
Hartel I, Patel J, Levinson J, Ferguson S, Campbell C, Weber A, Gomez S, Gazeley D, Duarte-Garcia A, Barton J, Garg S. “Prednisone… A Necessary Evil…” Developing an Evidence-Based Benefits vs. Harms Shared Decision-Making Tool (PRED-SAFE) to Support Decisions Around Chronic Prednisone Use [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/prednisone-a-necessary-evil-developing-an-evidence-based-benefits-vs-harms-shared-decision-making-tool-pred-safe-to-support-decisions-around-chronic-prednisone-u/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prednisone-a-necessary-evil-developing-an-evidence-based-benefits-vs-harms-shared-decision-making-tool-pred-safe-to-support-decisions-around-chronic-prednisone-u/
