Session Information
Date: Monday, October 27, 2025
Title: (0955–0977) Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is characterized by tissue fibrosis, which is defined as excessive and irreversible extracellular matrix (ECM) accumulation, leading to organ dysfunction. In SSc, ECM production is thought to be initiated by an inflammatory trigger. However, the precise kinetics of the inflammatory and fibrotic processes remain poorly described. Moreover, data regarding the independence of fibrosis from the initial inflammatory stimulus are lacking. This study aims to characterize the timing and interdependence of the inflammatory and fibrosis processes using an extended experimental mouse model of SSc.
Methods: Experimental SSc was induced by daily hypochlorous acid (HOCl) injections into the backs of mice for 9 weeks. Mice (n = 8-12 per group) were sequentially sacrificed at day (D)7, D14, D21, D28, D35, D42 and D63. Moreover, a group of mice receiving HOCl injections until D42 was sacrificed at D63 (group D42+21), to study the evolution of fibrosis after stopping HOCl injections. Skin architectural changes and ECM accumulation were assessed by histological approaches. Leukocyte count and distribution were analyzed by flow cytometry. The dynamic progression of the skin inflammatory and fibrotic functional program was assessed by RT-qPCR using a panel of 22 representative genes.
Results: Mice treated with HOCl showed early dermal thickening and collagen accumulation starting from D14 and D7, respectively, and progressing to a plateau by D28 (Figure 1A). HOCl injections impacted skin inflammatory cells in terms of number (higher number and percentage compared to control mice), localization (progressive infiltration from deep to superficial skin layers) and subset distribution (increase in NK et T cells percentages, no difference for myeloid cells or B cells, Figure 1B). Genes associated with inflammation were overexpressed in the skin from D7 to D42. Genes encoding ECM components were also upregulated starting from D7, with distinct peak expression times between type I collagen, type V collagen, and fibronectin (Figure 1C). Three weeks after stopping HOCl injections, no decrease in dermal thickness or collagen accumulation was observed (Figure 2A). Inflammation regressed, with a significant decrease in the expression of inflammatory cytokine genes (Ccl2, Cxcl1, Il6 and Il1b). A significant modification of the ECM component gene expression profile (decrease in Col1a1 and Col5a1 expression and increase in Col7a1 expression) and a drop in the expression of myofibroblast differentiation genes were noted (Figure 2B).
Conclusion: Inflammatory and fibrotic parameters are closely intertwined in the HOCl model, with both processes appearing from the onset of HOCl injections. After cessation of the inflammatory trigger, progressive ECM remodeling is observed, though without a return to baseline. A better understanding of the temporal dynamics of these processes in this experimental model may elucidate inflammation and fibrosis resolution mechanisms in SSc and guide the optimal timing for evaluating anti-inflammatory and anti-fibrotic therapies, both in preclinical studies and clinical translation.
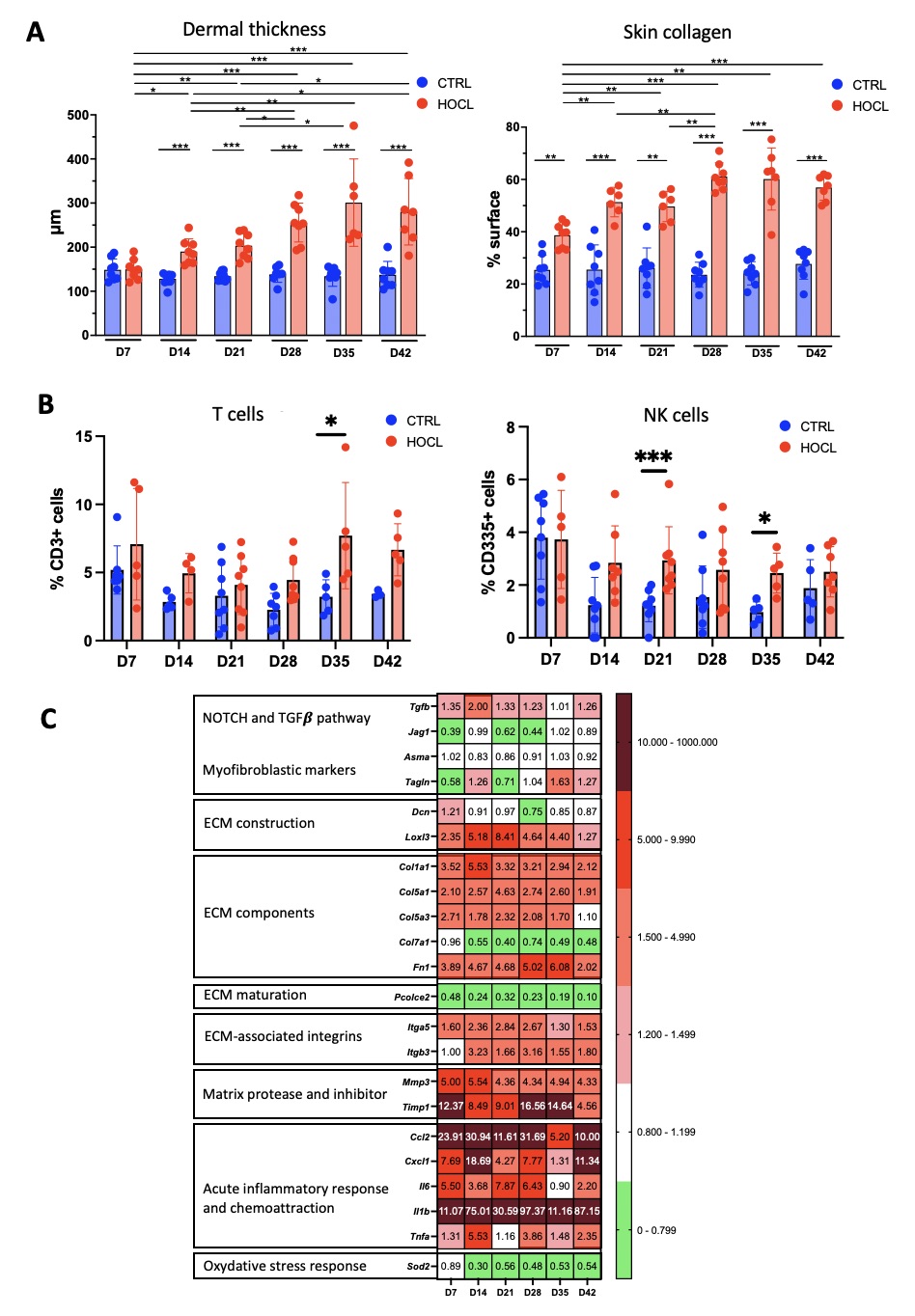 Figure 1: Kinetic evolution of skin inflammation and fibrosis in the HOCl-induced systemic sclerosis mouse model
Figure 1: Kinetic evolution of skin inflammation and fibrosis in the HOCl-induced systemic sclerosis mouse model
(A) Evolution of dermal thickness (in μm, on Hematoxylin-Eosin stained histological sections) and collagen content (% per section, on Picrosirius-Red stained histological sections). Results presented as mean +/- SD, N = 8 per group.
(B) Evolution of T cells and NK cells percentages among skin leukocytes, measured by flow cytometry. Results presented as mean +/- SD, n = 5-8 per group.
(C) Expression of genes representative of inflammatory and fibrosis processes in the skin. Heat map expressed as log2 fold change of gene expression measured by qPCR in HOCl mice, normalized on PBS mice with 𝜷actin gene as internal control. N = 12 per group.
* : P < 0,05 ; ** : P < 0,01 ; *** : P < 0,001.
.jpg) Figure 2: Evolution of skin inflammation and fibrosis after stopping the inflammatory trigger in the HOCl-induced systemic sclerosis mouse model
Figure 2: Evolution of skin inflammation and fibrosis after stopping the inflammatory trigger in the HOCl-induced systemic sclerosis mouse model
(A) Evolution of dermal thickness (in μm, on Hematoxylin-Eosin stained histological sections) and collagen content (% per section, on Picrosirius-Red stained histological sections). Results presented as mean +/- SD, N = 12 per group.
(B) Expression of genes representative of inflammatory and fibrosis processes in the skin. Heat map expressed as log2 fold change of gene expression measured by qPCR in HOCl mice, normalized on PBS mice with 𝜷actin gene as internal control. N = 12 per group.
** : P < 0,01 ; **** : P < 0,0001.
To cite this abstract in AMA style:
Collet A, Jendoubi M, Guerrier T, Largy A, Speca S, Dubucquoi S, Launay D. Asynchronous Resolution of Inflammation and Fibrosis in A Prolonged Experimental Model Suggests Distinct Temporal Dynamics And Resolution Mechanisms in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/asynchronous-resolution-of-inflammation-and-fibrosis-in-a-prolonged-experimental-model-suggests-distinct-temporal-dynamics-and-resolution-mechanisms-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/asynchronous-resolution-of-inflammation-and-fibrosis-in-a-prolonged-experimental-model-suggests-distinct-temporal-dynamics-and-resolution-mechanisms-in-systemic-sclerosis/
