Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-neutrophil cytoplasmic antibodies (ANCA), including those directed to myeloperoxidase (MPO) and proteinase 3 (PR3) as well as antibodies against anti-glomerular basement membrane (anti-GBM) are critical markers in the diagnosis of small vessel vasculitides and anti-GBM disease, respectively. While some multiplex platforms are available, conventional testing often involves sequential single-analyte assays, which may delay diagnosis and increase workload. There remains a clinical need for fully automated multiplexed assays capable of simultaneously detecting these key markers. This multicenter study evaluated the analytical performance of a novel, fully automated multiplexed microarray immunoassay prototype (MosaiQ AiPlex® VAS microarray; AliveDx, Switzerland), designed for the simultaneous detection of IgG autoantibodies to PR3, MPO, and GBM, in comparison with routine singleplex and multiplex methods.
Methods: The study was conducted at four sites in the United Kingdom, including three public reference laboratories (two located in England and one in Wales) and a sponsor’s site in Scotland. Comparator methods varied by site and included fluorescence enzyme immunoassay (FEIA), addressable laser bead immunoassay (ALBIA) and chemiluminescent immunoassay (CLIA). De-identified, banked serum samples previously characterized as reactive or non-reactive for PR3, MPO or GBM antibodies were tested using the investigational microarray prototype on its proprietary automated platform. Analytical performance was assessed by calculating positive percent agreement (PPA), negative percent agreement (NPA) and overall percent agreement (OPA) both in comparison to each site’s routine method and in aggregate across all sites and platforms. One site using CLIA did not have anti-GBM reactive samples available for testing.
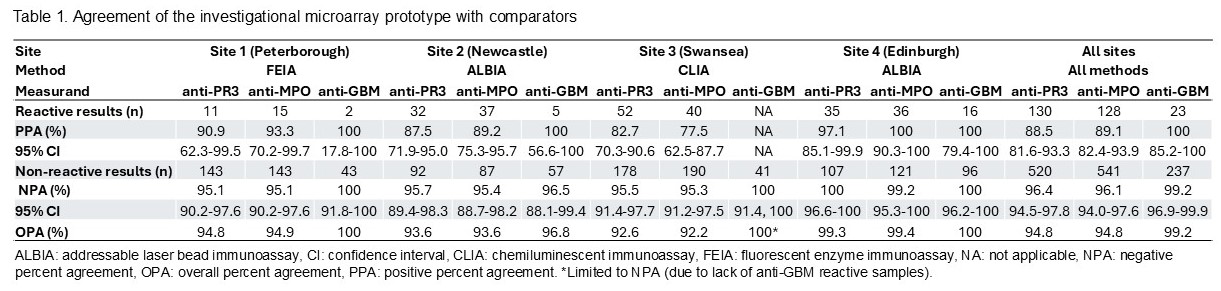
Results: The investigational microarray prototype consistently demonstrated high NPA across all measurands and comparator methods, ranging from 95.1% (anti-PR3 and anti-MPO, FEIA) to 100% (anti-PR3 at 1 ALBIA site and anti-GBM with FEIA, CLIA and at 1 ALBIA site). Overall NPA was 96.4% for anti-PR3 (95% CI: 94.5, 97.8), 96.1% for anti-MPO (95% CI: 94.0, 97.6) and 99.2% for anti-GBM (95% CI: 96.9, 99.9). PPA ranged from 77.5% (anti-MPO, CLIA) to 100% (anti-GBM across methods). Overall PPA across sites and methods was 88.5% for anti-PR3 (95% CI: 81.6, 93.3), 89.1% for anti-MPO (95% CI: 82.4, 93.9) and 100% for anti-GBM (95% CI: 85.2, 100). OPA across sites and methods ranged from 94.8% to 99.2%. Detailed results are summarized in Table 1.
Conclusion: In this multicenter study, the investigational microarray prototype showed high concordance with routine singleplex and multiplex methods for the detection of anti-PR3, anti-MPO, and anti-GBM autoantibodies. These findings support the potential utility of the assay as a single-platform solution for streamlined, simultaneous serologic evaluation of ANCA-associated vasculitis and anti-GBM disease. Future studies incorporating clinically characterized samples will enable expanded assessment of the assay’s diagnostic performance.
To cite this abstract in AMA style:
Swierczynska N, Armour R, Yau A, Rusi E, Hooper M, Sillitoe J, Wilson C, Griffiths P, El-Shanawany T, Sims F, Gomez G, Fischer C, Hausmann M, Laird H. Analytical Performance of a Novel, Fully Automated Multiplexed Microarray Immunoassay Prototype for the Simultaneous Detection of Autoantibodies to GBM, PR3, and MPO: A Multicenter Evaluation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/analytical-performance-of-a-novel-fully-automated-multiplexed-microarray-immunoassay-prototype-for-the-simultaneous-detection-of-autoantibodies-to-gbm-pr3-and-mpo-a-multicenter-evaluation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analytical-performance-of-a-novel-fully-automated-multiplexed-microarray-immunoassay-prototype-for-the-simultaneous-detection-of-autoantibodies-to-gbm-pr3-and-mpo-a-multicenter-evaluation/