Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Giant cell arteritis (GCA) relapses are frequent and often require therapeutic intensification in the form of glucocorticoids (GC) increase. GCA management has significantly evolved following the publication of the GiACTA trial and the subsequent approval of tocilizumab (TCZ) in 2017, allowing for faster GC tapering without relapse. In this study, we aimed to describe real-world diagnostic practices, management strategies and mortality outcome in patients with new-onset GCA from 2017 to 2024.
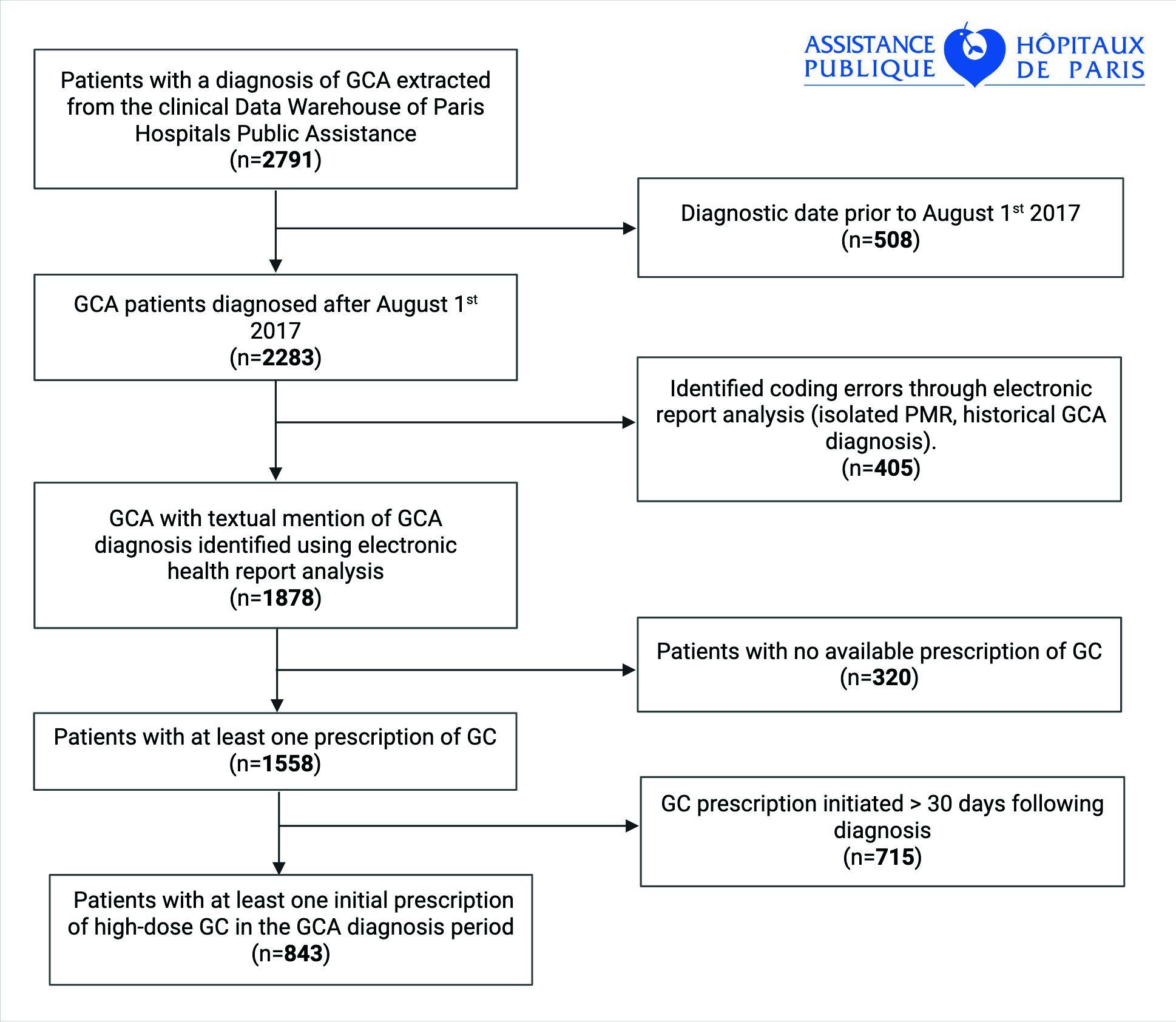
Methods: All patients diagnosed GCA between August 1st 2017 and May 30th 2024, were identified in the Clinical Data Warehouse of Assistance Publique–Hôpitaux de Paris using the French Medical Information System Program coding system. Stored data integrates clinical, biological and imaging data from patients hospitalized across all public hospitals in the Greater Paris area. To improve cohort accuracy, a natural language processing algorithm was applied to detect and exclude miscoded entries. Patients without a recorded prescription for GC were excluded. The study flowchart is presented in Figure 1. The risk of death was assessed using a multivariate Cox proportional hazards model.
Results: A total of 843 patients were included in the study, 543 (64%) of whom were female, with a median [interquartile range] age at diagnosis of 74 [68–81] years, and median follow-up 6.4 [3.0-9.9] years (Table 1). The use of positron emission tomography increased from 30.4% of patients in the year 2017, to 36.5% in the year 2024. Conversely, the proportion of temporal artery biopsies declined from 47.8% in 2017, compared to 38.5% in the year 2024. At diagnosis, 772 (91.6%) received GC monotherapy while 71 (8.4%) received combined therapy with TCZ and GC. During follow-up, 400 (47.5%) GCA patients required GC intensification, including 319 (38%) at 1 year, 363 (43%) at 2 years and 380 (45%) at 3 years. The median time to first GC intensification was 1.4 [0.1–5.6] years. The median GC dose before intensification was 10 [5-20] mg/day which increased to 35 [20-50] mg/day following intensification. Overall, 91 (11%) patients died during follow-up. In a multivariate analysis (Table 2), older age at diagnosis (p< 0.01) and the presence of a stroke at baseline (p< 0.01) were independently associated with an increased risk of mortality.
Conclusion: In this contemporary French cohort, nearly half of patients with GCA required at least one GC intensification during the course of treatment. Stroke at baseline and older age at diagnosis were identified as independent risk factors for all-cause mortality.
Abbreviations: GCA: giant cell arteritis; GC: glucocorticoids; PMR: polymyalgia rheumatica.
.jpg) Table 1. Clinical characteristics at GCA diagnosis.
Table 1. Clinical characteristics at GCA diagnosis.
Abbreviations: GCA: giant cell arteritis; CT: computed tomography; PET : positron emission tomography
* Comorbidities at baseline were defined as clinical history or concomitant to GCA diagnosis.
.jpg) Table 2. Survival analysis for risk of death among GCA patients.
Table 2. Survival analysis for risk of death among GCA patients.
Results of both univariate and multivariate survival analysis for the risk of death following GCA diagnosis, using a Cox proportional hazard model. Comorbidities at baseline were defined as clinical history or concomitant to GCA diagnosis.
Abbreviations: CI: confidence interval; GCA: giant cell arteritis; HR: hazard ratio
To cite this abstract in AMA style:
Kante A, Hassanaly O, Peyrac G, Saadoun D, Patrice C, REGENT A, Terrier B, Mouthon L, Mekinian A, Sacré K, Alexandra J, Abad S, Dhote R, goujard C, Sène D, Mouly S, Hervier B, Bory O, Aslangul E, Mahé I, Couvelard A, Nguyen Y, Lefort A, georgin-Lavialle S, Steichen O, Tran V, Comarmond C. A Real-World Study from the Greater Paris Clinical Data Warehouse [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-real-world-study-from-the-greater-paris-clinical-data-warehouse/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-real-world-study-from-the-greater-paris-clinical-data-warehouse/