Session Information
Date: Sunday, October 26, 2025
Title: (0430–0469) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: A third of patients with early (e)RA report pain outside the joint or non-articular pain (NAP) despite RA treatment(1). NAP, both regional and widespread, impairs HR-QOL and negatively impacts remission outcomes in eRA(1). Patients with eRA report having NAP in both upper body areas nearly twice as often as both lower body areas(1). How NAP, both regional and widespread, impacts physical function, including that of upper extremities, is not well understood. We aim to evaluate the impact of NAP, regional and widespread, on physical function using 3 instruments 1) Multi-Dimensional Health Assessment Questionnaire (MDHAQ) 2) PROMIS29 Physical Function (PF) 3) Neuro-QOL Upper Extremity (UE) Function (assesses reach function and ADLs) (2), at baseline and over the first year of RA diagnosis and treatment.
Methods: Data were from patients with active early RA (symptoms< 1 year, CDAI >2.8) enrolled in the Canadian Early Arthritis Cohort between Jan/2017-10/2023. Patients were instructed to indicate any non-joint pain they experienced on a body pain diagram (BPD) at baseline (BL), and 6- and 12-month follow-up visits. Prespecified NAP patterns were classified based on pain reported in 5 sections (4 quadrants and axial, excluding hands and feet) and grouped as: 1) no NAP (no sections selected on BPD), 2) regional (1-3 sections) or 3) widespread NAP(3+sections). Patients completed synchronous assessments of physical function: MDHAQ (0-3), PROMIS29 PF and Neuro-QoL UE function T-scores (mean is 50, 1 standard deviation is 10) over 1-year. Adjusted associations between NAP and physical function were estimated in separate multivariable mixed effects models adjusted for baseline age, sex, education, smoking, comorbidities, seropositivity and time-variant CDAI.
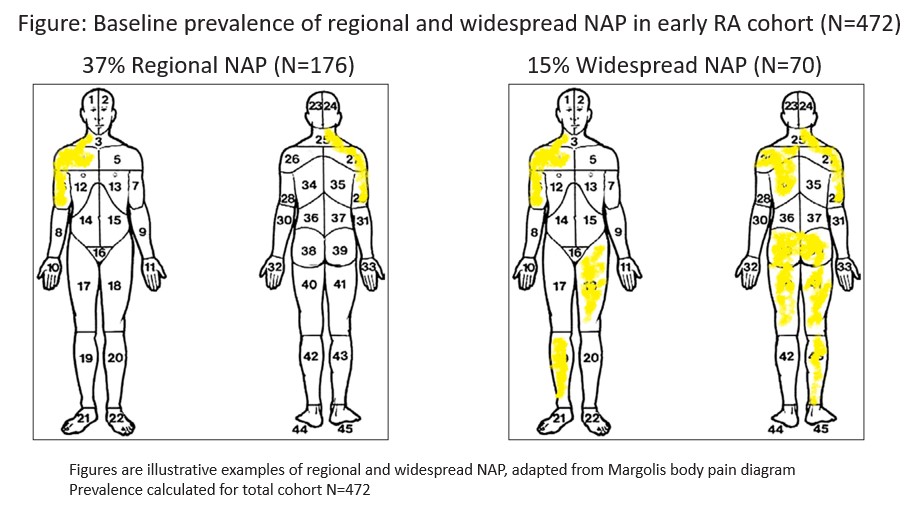
Results: The 472 eRA patients were mostly female (66%) with a mean (SD) age of 57(14) and had active disease at baseline, with a mean (SD) CDAI 27.0 (14.1) (Table 1). Most (79%) were treated with methotrexate. Regional NAP was more prevalent than widespread (Figure). Compared to other groups, those with widespread NAP more often had higher BMI, reported more comorbidities, smoked and had higher CDAIs. NAP was associated with worse baseline function assessed by MDHAQ, PROMIS29 PF and Neuro-QOL UE function in a dose-response manner across pain groups (none, regional and widespread NAP). The adjusted regression analyses similarly exhibited a dose-response effect of NAP on physical function with worsening in all 3 outcomes over 1-year (Table 2). All relationships were statistically significant. Neuro-QoL UE T-scores worsened by close to half a standard deviation in those with widespread NAP (Table 2).
Conclusion: NAP is common in eRA and negatively impacts physical function, including that of upper extremities, at baseline and over the first year of RA diagnosis and treatment. Worse physical function by all 3 instruments were observed with greater NAP distribution. Early identification and targeted intervention of NAP is recommended to help prevent long-term disability in patients with RA.1.Meng C. Characterizing NAP at early RA diagnosis. A&R. 2025 Apr;77(4):405-413.2.Bartlett SJ. The Neuro-QOL upper extremity function scale. ARD. 2021;80(Suppl 1):159-60.
To cite this abstract in AMA style:
Meng C, Valois M, Julia C, Lee Y, Kuriya B, Boire G, Allard-Chamard H, Hitchon C, Bessette L, Hazlewood G, Thorne C, Bartlett S, Pope J, Bykerk V. Beyond the Joints: The Impact of Non-Articular Pain on Patient-Reported Function in a Longitudinal Real-World Early RA Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/beyond-the-joints-the-impact-of-non-articular-pain-on-patient-reported-function-in-a-longitudinal-real-world-early-ra-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/beyond-the-joints-the-impact-of-non-articular-pain-on-patient-reported-function-in-a-longitudinal-real-world-early-ra-cohort/

.jpg)
.jpg)