Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Modern therapies have improved outcomes in patients with JIA, but up to 60% of patients treated with TNF-α inhibitors (TNFi) have persisting disease activity underscoring the need for improved therapeutical approaches. Intra-articular (IA) triamcinolone hexacetonide injections are often used as adjunctive therapy to TNFi treatment even though no controlled studies have examined their potential added benefit. We aim to assess the efficacy and safety of IA triamcinolone hexacetonide injections compared to no injections in children with JIA starting TNFi therapy.
Methods: The MyJIA trial (NCT 04614311) is a randomized, controlled, multicenter, parallel group, blinded-assessor, open-label, phase 4 clinical trial. Main inclusion criteria were non-systemic JIA, age 1 to 18 years, clinical indication for TNFi therapy, and at least one joint with arthritis suited for IA steroid injection. Participants were assigned by a computer randomization procedure (1:1) to IA triamcinolone hexacetonide injections of active joints or no joint injections. Core outcome variables for JIA were collected at baseline, weeks 6, 12, 24, 36 and 48. Participants randomized to IA injections had joints with arthritis injected at baseline and if clinically indicated at follow-up visits with triamcinolone hexacetonide (large joints 0.5-1 mg/kg (max 40mg), medium joints 0.3-0.5 (max 20mg), small joints, 0.1-0.3 (max 15mg), maximum total dose per visit 3.5mg/kg or < 140mg). Primary endpoint was the proportion of patients in sustained inactive disease, as defined by the JIA-ACR/Wallace 2011 criteria, at both weeks 24 and 36, analysed using logistic regression adjusted for study site.
Results: In total 187 children with non-systemic JIA were randomized; 92 (49%) to the injection group, and 95 (51%) to the no-injection group. Baseline characteristics are summarized in Table 1. The primary endpoint, the proportion of patients achieving JIA-ACR inactive disease at weeks 24 to 36, was 35% in the IA steroid injection group compared to 33% in the no-injection group (risk difference (RD) 0.02, 95% CI -0.12-0.15, p 0.80) (Figure 1). The mean time to JIA-ACR inactive disease was similar between groups (mean difference -4.5 days, 95% CI -37.4-28.5). JIA-ACR30 response rates were significantly higher in the IA steroid injection group when compared to the no-injection group with a risk difference of 12% (95% CI 0.01-0.24, p=0.03) at week 6, 16% at week 12 (95% CI 0.06-0.26, p< 0.01), 9% (95% CI 0.01-0.17, p=0.03) at week 24, and 6% (95% CI 0.00-0.13, p=0.04) at week 36. At week 48, JIA-ACR30 response rates were similar (RD 0.03, 95% CI -0.01-0.08, p=0.08). (Figure 1). JIA-ACR 70/90 and JIA-ACR core set variables showed no between-group differences at any time point. AEs/SAEs occurred in 67%/7% in the injection group, and in 75%/8% in the no-injection group. Subcutaneous atrophy occurred in 8 of 288 steroid injected joints (3%).
Conclusion: IA triamcinolone hexacetonide injections did not increase the proportion of JIA patients with sustained inactive disease up to 48 weeks after starting TNFi therapy. These findings show that added intra-articular steroid injections is redundant in children with JIA starting TNFi therapy.
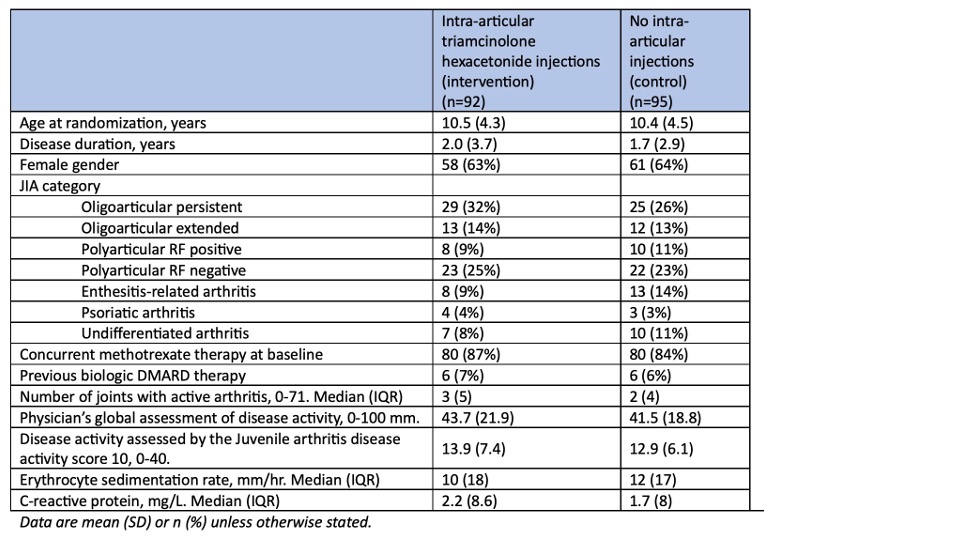 Table 1. Baseline demographics and disease characteristics.
Table 1. Baseline demographics and disease characteristics.
.jpg) Figure 1. Efficacy of intra-articular triamcinolone hexacetonide injections in juvenile idiopathic arthritis (JIA) patients starting tumor necrosis factor inhibitor (TNFi) therapy.
Figure 1. Efficacy of intra-articular triamcinolone hexacetonide injections in juvenile idiopathic arthritis (JIA) patients starting tumor necrosis factor inhibitor (TNFi) therapy.
A) Primary endpoint: Bar chart of the proportion of patients in sustained JIA-ACR/Wallace inactive disease at both weeks 24 and 36 for patients receiving intra-articular steroid injections versus no injections (n=187). Estimates (95% CI) from logistic regression model adjusted for study center. B) JIA-ACR inactive disease response rates with estimates (95% CIs) from logistic mixed model including weeks 6, 12, 24, 36 and 48 by treatment group (n=163). C) JIA-ACR30, 50, 70, and 90 response rates with estimates (95% CIs) from logistic mixed model including weeks 6, 12, 24, 36 and 48 by treatment group (n=163).
To cite this abstract in AMA style:
Büyesen P, Aga A, Lilleby V, Pesonen M, Rygg M, Nordal E, Barstad B, Tylleskär K, Sanner H, Hetlevik S, Sande N, Olsen I, Lillegraven S, Haavardsholm E, Ramanan A, Molberg O, Flatø B. Long-term efficacy of intra-articular triamcinolone hexacetonide injections in juvenile idiopathic arthritis patients starting tumor necrosis factor inhibitor therapy: 48 weeks results from a randomized, open-label, blinded-assessor multicenter phase 4 trial – the MyJIA trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-efficacy-of-intra-articular-triamcinolone-hexacetonide-injections-in-juvenile-idiopathic-arthritis-patients-starting-tumor-necrosis-factor-inhibitor-therapy-48-weeks-results-from-a-randomiz/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-of-intra-articular-triamcinolone-hexacetonide-injections-in-juvenile-idiopathic-arthritis-patients-starting-tumor-necrosis-factor-inhibitor-therapy-48-weeks-results-from-a-randomiz/
