Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Administrative claims databases enable research in large populations with JIA. We previously showed that machine learning (ML)-based algorithms accurately identify new JIA diagnoses within US commercial insurance claims data. We externally validated these algorithms within US public insurance claims data.
Methods: We performed a cross-sectional validation study using US commercial health plan data (2013-20) and national US Medicaid data (2013-18). We identified children diagnosed with JIA (ICD-9-CM: 696.0, 714, 720; ICD-10-CM: L40.5, M05, M06, M08, M45) before age 18 after ≥12 months’ continuous enrollment without JIA diagnosis or immunosuppression. JIA diagnoses were based on 3 previously validated definitions: 1) rheumatologist’s diagnosis plus ≥2 specific lab test orders; 2) ≥2 outpatient diagnoses 8-52 weeks apart; or 3) 1 inpatient diagnosis. A random set of available qualifying charts were abstracted and independently adjudicated as definite, probable, possible, or unlikely JIA by clinical experts; discrepancies were resolved by consensus. Incident JIA was defined as definite or probable JIA diagnosed ≤4 months before first JIA claim. ML-based algorithms used simulation-based balancing and logistic regression regularization hyperparameters with 10-fold cross-validation. We used optimal predictive model variables to assess sensitivity (Se), specificity (Sp), and positive predictive value (PPV) (95% confidence interval [CI]). We also tested rule-based algorithms refined based on provider type, JIA diagnosis counts, laboratory test counts, and documented JIA treatment. We compared results across databases and ICD types.
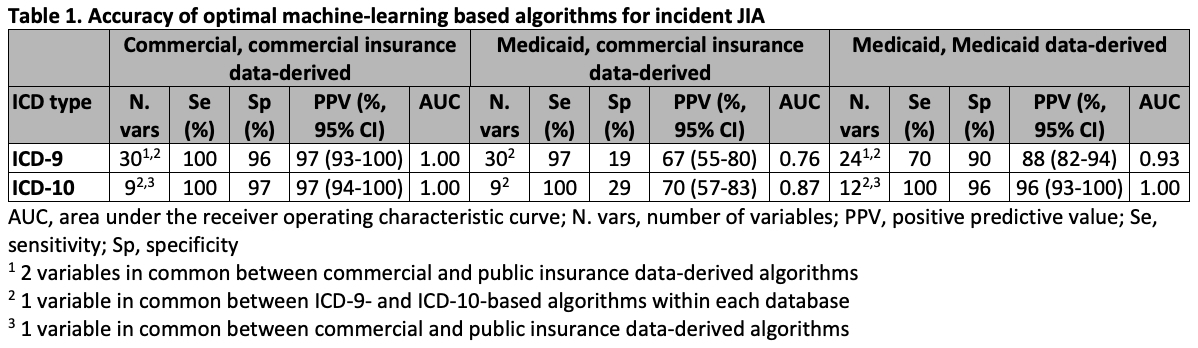
Results: Of 298 eligible charts reviewed (182 commercial, 116 public), 151 had incident JIA (ICD-9 commercial 58%, public 53%; ICD-10 commercial 41%, public 52%). Optimal ML-based algorithms derived within commercial claims data enabled excellent discrimination between incident JIA and unlikely JIA (ICD-9: Se 100%, Sp 96%, PPV 97%; ICD-10: Se 100%, Sp 97%, PPV 97%) (Table 1). However, the same algorithm was not accurate within the Medicaid sample (ICD-9: Se 97%, Sp 19%, PPV 67%; ICD-10: Se 100%, Sp 29%, PPV 70%), and more accurate algorithms derived within Medicaid data used distinct sets of predictive variables (Table). Moreover, optimal ML-based algorithms differed in number and types of predictors across ICD-9 and ICD-10 data. Rule-based algorithms had lower specificity and/or sensitivity, but refined algorithms were more accurate and consistent across databases and ICD types (Table 2-3). Preferred rule-based algorithms required either: 1) rheumatologist’s outpatient diagnosis plus ≥4-5 specific lab orders, or 2) ≥5 outpatient JIA visits (first diagnosis not for eye care) plus any JIA treatment.
Conclusion: While ML-based diagnostic algorithms for incident JIA performed well within each database and ICD type, results differed across databases and ICD types. In contrast, refined rule-based algorithms had better external validity, with similarly high PPVs across databases and ICD code types. These preferred rule-based algorithms will improve the quality of future claims-based research on the diagnosis, management, and outcomes of newly diagnosed JIA.
To cite this abstract in AMA style:
Horton D, Parlett L, Zhu Y, Rege S, Hoffman P, Reiff D, McGuire S, Pothraj S, Salvant C, Moorthy L, Huang C, Koffman D, Iozzio M, Iizuka A, Schott K, Crystal S, Davidow A, Gerhard T, Haynes K, Strom B, Beachler D, Rose C. External Validation of Claims-based Algorithms for Newly Diagnosed Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/external-validation-of-claims-based-algorithms-for-newly-diagnosed-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/external-validation-of-claims-based-algorithms-for-newly-diagnosed-juvenile-idiopathic-arthritis/

.jpg)
.jpg)