Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Dermatomyositis (DM) is a major subtype of idiopathic inflammatory myopathies (IIMs) and characterized by muscle weakness, systemic inflammation and cutaneous lesions. Expression of type I interferon (IFN)-stimulated genes have been found in muscle, skin tissue and peripheral blood cells which links to disease activity in DM patients. However, the mechanisms of muscle weakness in DM are incompletely understood. For instance, it is unclear to what degree systemic factors, eg IFN signalling, contribute to muscle weakness and disease phenotypes. This study aims to investigate whether inhibition of type I IFN signaling protects DM serum-induced muscle weakness and to determine the effects of IFN-a and/or IFN-b on muscle force generation.
Methods: An experimental platform was used in which isolated mouse muscles were exposed to DM patient serum or healthy control serum. Flexor digitorum brevis (FDB) muscles were isolated from healthy C57BL/6JRj mice and exposed to 10% dilution of serum from healthy controls or serum from DM patients for 24 hours. To determine whether inhibition of type I IFN protect DM serum-induced muscle weakness, an IFN-a/b receptor 1 (IFNR) blocking antibody was used. Isolated FDB muscles were pre-incubated with IFNAR1 antibody for 2 h and thereafter muscles were incubated in serum from DM patients with or without IFNAR1 antibody. To investigate the effect of IFN-a and/or IFN-b on muscle force generation, FDB muscles were incubated in 10% healthy control serum containing of 1,000U/ml of IFN-a and/or IFN-b. Muscle force was measured before and after incubation in a paired fashion.
Results: Serum from DM patients, but not from healthy controls, induced a decline in FDB muscle force. Treatment with an anti-IFN1R antibody protected from the DM serum-induced muscle force decline. Surprisingly, neither IFN-a nor IFN-b affected force production when incubated for 24h in isolation with the muscles. However in combination, IFN-a together with IFN-b, induceed a marked reduction in muscle force, which was rescued with addition of an IFN1R blocking antibody.
Conclusion: Serum from DM patients contain factors that can induce muscle weakness. One mechanism for this effect is mediated by pathways dependent on type I IFN Receptor signaling.
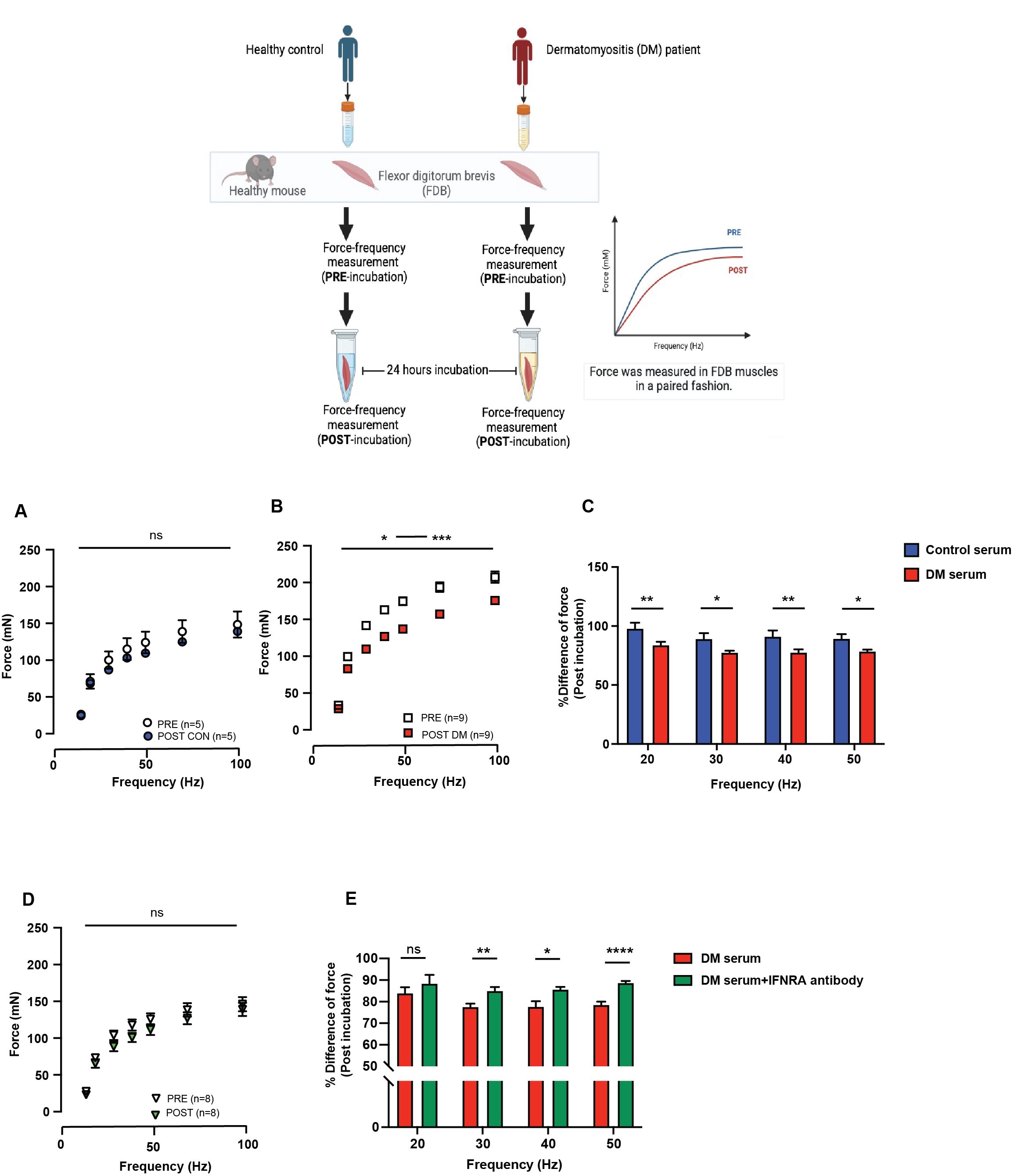 Figure 1 Inhibition of type I IFN pathway protects the effect of DM serum-induced muscle weakness in isolated skeletal muscles from healthy mice. (A) Force-frequency curve from isolated flexor digitorum brevis (FDB) muscles before (PRE) and after (POST) incubation with 10% serum from healthy control. Isolated FDB muscles were incubated with serum from healthy control and repeated in difference muscles for five times. (B) Force-frequency curve from FDB muscles before (PRE) and after (POST) incubation with 10% serum from dermatomyositis (DM) patients. Serum from nine DM patients were used in the experiments. Force measurement was performed in isolated FDB muscle in a paired fashion. (C) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy control (blue) or DM serum (red) at 20-50 Hz. (D) Force-frequency curve from FDB muscles were incubated with serum form DM patients in the presence of IFNAR1 antibody. (E) The bar graph demonstrates the percentage of differences of force after exposed to serum from DM patients (red) or DM serum with IFN-/ receptor 1 (IFNAR1) antibody (dark green) at 20-50 Hz. Data were presented as mean SEM, *p < 0.05, **p < 0.01 ***p < 0.0001, Bonferroni multiple comparison test was performed for data analysis.
Figure 1 Inhibition of type I IFN pathway protects the effect of DM serum-induced muscle weakness in isolated skeletal muscles from healthy mice. (A) Force-frequency curve from isolated flexor digitorum brevis (FDB) muscles before (PRE) and after (POST) incubation with 10% serum from healthy control. Isolated FDB muscles were incubated with serum from healthy control and repeated in difference muscles for five times. (B) Force-frequency curve from FDB muscles before (PRE) and after (POST) incubation with 10% serum from dermatomyositis (DM) patients. Serum from nine DM patients were used in the experiments. Force measurement was performed in isolated FDB muscle in a paired fashion. (C) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy control (blue) or DM serum (red) at 20-50 Hz. (D) Force-frequency curve from FDB muscles were incubated with serum form DM patients in the presence of IFNAR1 antibody. (E) The bar graph demonstrates the percentage of differences of force after exposed to serum from DM patients (red) or DM serum with IFN-/ receptor 1 (IFNAR1) antibody (dark green) at 20-50 Hz. Data were presented as mean SEM, *p < 0.05, **p < 0.01 ***p < 0.0001, Bonferroni multiple comparison test was performed for data analysis.
.jpg) IFN-a and IFN-b decline contractile force in isolated FDB muscle. (A) Force-frequency curve from FDB muscle before (PRE) and after (POST) incubated with 10% healthy serum containing IFN-a (1,000U/ml) for 24 hours. (B) Force-frequency curve from FDB muscle before (PRE) and after (POST) exposed to 10% healthy serum containing IFN-b (1,000U/ml). (C) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy serum containing of IFN-a (red) or IFN-b (blue) and combination of IFN-a plus IFN-b (green) at 20-50 Hz. (D) Force-frequency curve from FDB muscle before (PRE) and after (POST) exposed to 10% of healthy serum containing IFN-a together with IFN-b (1,000U/ml). (E) Force-frequency curves from FDB muscle before (PRE) and after (POST) incubated with 10% healthy serum containing of IFN-a and IFN-b (1,000U/ml) and treated with type I IFN-a/b receptor1 (IFN1R) antibody at the final concentration of 10g/ml (green). (F) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy serum containing of IFN-a and IFN-b (dark green) or IFN-a and IFN-b plus IFN1R blocking antibody (light green) at 20-50 Hz. Data were presented as mean +/-SEM, *p < 0.05, **p < 0.01 ***p < 0.0001, two way ANOVA, Bonferroni multiple comparison test was performed for the data analysis.
IFN-a and IFN-b decline contractile force in isolated FDB muscle. (A) Force-frequency curve from FDB muscle before (PRE) and after (POST) incubated with 10% healthy serum containing IFN-a (1,000U/ml) for 24 hours. (B) Force-frequency curve from FDB muscle before (PRE) and after (POST) exposed to 10% healthy serum containing IFN-b (1,000U/ml). (C) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy serum containing of IFN-a (red) or IFN-b (blue) and combination of IFN-a plus IFN-b (green) at 20-50 Hz. (D) Force-frequency curve from FDB muscle before (PRE) and after (POST) exposed to 10% of healthy serum containing IFN-a together with IFN-b (1,000U/ml). (E) Force-frequency curves from FDB muscle before (PRE) and after (POST) incubated with 10% healthy serum containing of IFN-a and IFN-b (1,000U/ml) and treated with type I IFN-a/b receptor1 (IFN1R) antibody at the final concentration of 10g/ml (green). (F) The bar graph demonstrates the percentage of differences of force after exposed to serum from healthy serum containing of IFN-a and IFN-b (dark green) or IFN-a and IFN-b plus IFN1R blocking antibody (light green) at 20-50 Hz. Data were presented as mean +/-SEM, *p < 0.05, **p < 0.01 ***p < 0.0001, two way ANOVA, Bonferroni multiple comparison test was performed for the data analysis.
To cite this abstract in AMA style:
Kaewin S, Leijding C, Andreasson K, Alexanderson H, Gastaldello S, Lundberg I, Andersson D. Sera from dermatomyositis patients induce muscle weakness via activation of type I interferon (IFN) receptors. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sera-from-dermatomyositis-patients-induce-muscle-weakness-via-activation-of-type-i-interferon-ifn-receptors/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sera-from-dermatomyositis-patients-induce-muscle-weakness-via-activation-of-type-i-interferon-ifn-receptors/
