Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Progressive Pulmonary Fibrosis (PPF) is an increasingly recognized condition, defined in 2022 to address the progression of pulmonary fibrosis in patients with interstitial lung diseases (ILDs) other than idiopathic pulmonary fibrosis (IPF). PPF is a characteristic feature of autoimmune ILD. Oral pirfenidone has shown positive signals in some studies without reaching the primary endpoint, but treatment with oral pirfenidone is also associated with significant adverse events and a poor tolerability profile. This study aims at exploring the efficacy and safety of inhaled pirfenidone which might be a promising therapeutic approach considering the local drug delivery into the lungs and the subsequent reduced systemic exposure. Data from the AP01-002 (ATLAS) Study of inhaled pirfenidone in IPF demonstrated efficacy and improved safety compared to that seen historically with oral pirfenidone. The AP01-005 long term extension study allowed new patients with PPF to enter the study and showed a trend toward FVC stabilization. The AP01-007 (MIST Study) is designed to study the efficacy and safety of AP01 (inhaled pirfenidone) in patients with PPF including autoimmune ILD patients.
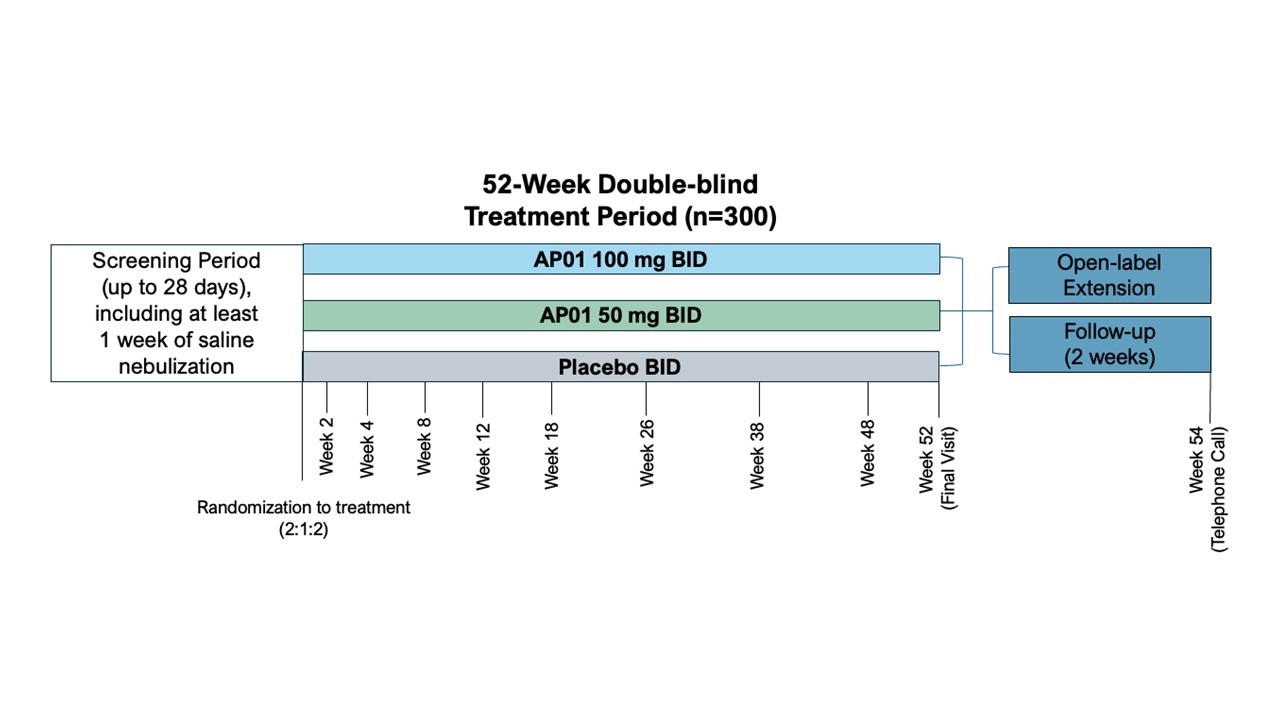
Methods: MIST is a prospective randomized placebo-controlled trial comparing two doses (100mg BID and 50mg BID) of inhaled pirfenidone with placebo over 52 weeks. Patients will be eligible if they meet at least one of the following criteria for progression of ILD within the 24 months prior to screening: a relative decline in the FVC of at least 10% of the predicted value, a relative decline in the FVC of ≥5% to less than < 10% of the predicted value accompanied by worsening of respiratory symptoms or an increased extent of fibrosis on HRCT; or worsening of respiratory symptoms and an increased extent of fibrosis on HRCT. Patients will remain on stable background immunosuppression, and up to 30% of patients will remain on background nintedanib therapy. 300 patients will be recruited in 14 countries, including the US.
Results: The AP01-007 (MIST Study) is designed to study the efficacy and safety of AP01 (inhaled pirfenidone) in patients with PPF including autoimmune ILD patients. The primary endpoint is the change in the annual rate of decline in FVC in the AP01 treatment groups as compared to placebo. In addition, efficacy will be measured in secondary endpoints of time to progression, change in fibrotic scores via quantitative HRCT, and change from baseline QoL. Safety outcomes will be assessed as well. Cough will be analyzed through cough questionnaires, which should allow differentiation of cough related to PPF vs. aerosol inhalation via nebulization.
Conclusion: MIST is the first study to analyze the novel concept of inhaled medications in autoimmune ILD. In addition to the safety and efficacy endpoints, MIST will examine the presence of cough in this population of patients.
To cite this abstract in AMA style:
Highland K, Reisner C, Trucillo A, Nair D, Woodhead F, Lazarus H, Conoscenti C, Kolb M. Inhaled pirfenidone as an innovative therapeutic approach to treat autoimmune ILD and other forms of Progressive Pulmonary Fibrosis: Phase 2b Study Design [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/inhaled-pirfenidone-as-an-innovative-therapeutic-approach-to-treat-autoimmune-ild-and-other-forms-of-progressive-pulmonary-fibrosis-phase-2b-study-design/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhaled-pirfenidone-as-an-innovative-therapeutic-approach-to-treat-autoimmune-ild-and-other-forms-of-progressive-pulmonary-fibrosis-phase-2b-study-design/