Session Information
Date: Sunday, October 26, 2025
Title: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex disease that presents unique care challenges. To improve high-quality patient-centered care, the American College of Rheumatology (ACR), developed evidence-based quality measures related to using patient reported outcome measures (PROMs) in SLE. This study aimed: 1) To conduct qualitative interviews with practices that had implemented ACR-recommended SLE PROMs as part of the pilot evaluation, using the Consolidated Framework for Implementation Research (CFIR) and 2) To synthesize implementation findings to inform the development of the SLE Quality Measures Implementation Guide for national dissemination.
Methods: Using purposive sampling, we invited five community-based rheumatology practices representing geographically diverse clinical settings to pilot the routine collection of SLE PROMs, including measures of physical function (RAPID-3 or MDHAQ) and depression (PHQ-8). After the pilot, rheumatologists and medical staff from each practice participated in qualitative virtual interviews, guided by the CFIR, to identify barriers and facilitators to implementing SLE PROMs and explore resources needed to improve the collection and use of the measures in clinical care. Recorded interviews were transcribed and analyzed thematically using deductive and inductive techniques to identify themes. Subsequently, insights from interviews, combined with ACR guidelines and other resources, informed the development of an SLE Quality Measures Implementation Guide (Figure 1).
Results: Thirty-one clinicians engaged in the pilot evaluation across five practices (Table 1). Qualitative Interviews with representatives from each practice (Nf8) revealed key insights into the innovations, resources, recommendations, best practices, and challenges related to SLE PROMs collection and use (Table 2). Despite challenges with workflow integration, clinician and medical staff engagement, and limited resources, both patients and clinicians supported using PROMs in routine care. PROMs effectively assessed mental health and physical function through standardized workflows, enhanced communication, and EHR integration. These findings informed the development of an online SLE Quality Measures Implementation Guide, designed to aide clinicians and care teams with implementing the recommended measures into clinical practice. Sections include recommended detailed instructions for use of ACR-recommended SLE PROMs, downloadable PROMs, workflow recommendations and scoring guidance. Reflecting themes from interviews, the guide also includes videos on best practices, patient education materials, staff training resources, and validated translations of PROMs in Spanish and Chinese.
Conclusion: This study assessed the implementation of ACR-recommended SLE PROMs in five rheumatology practices and used the findings to develop the SLE Quality Measures Implementation Guide. This guide supports rheumatologists and care teams with integrating PROMs into routine practice by offering guidance and practical strategies for collecting the measures to guide clinical decisions and advance patient-centered care.
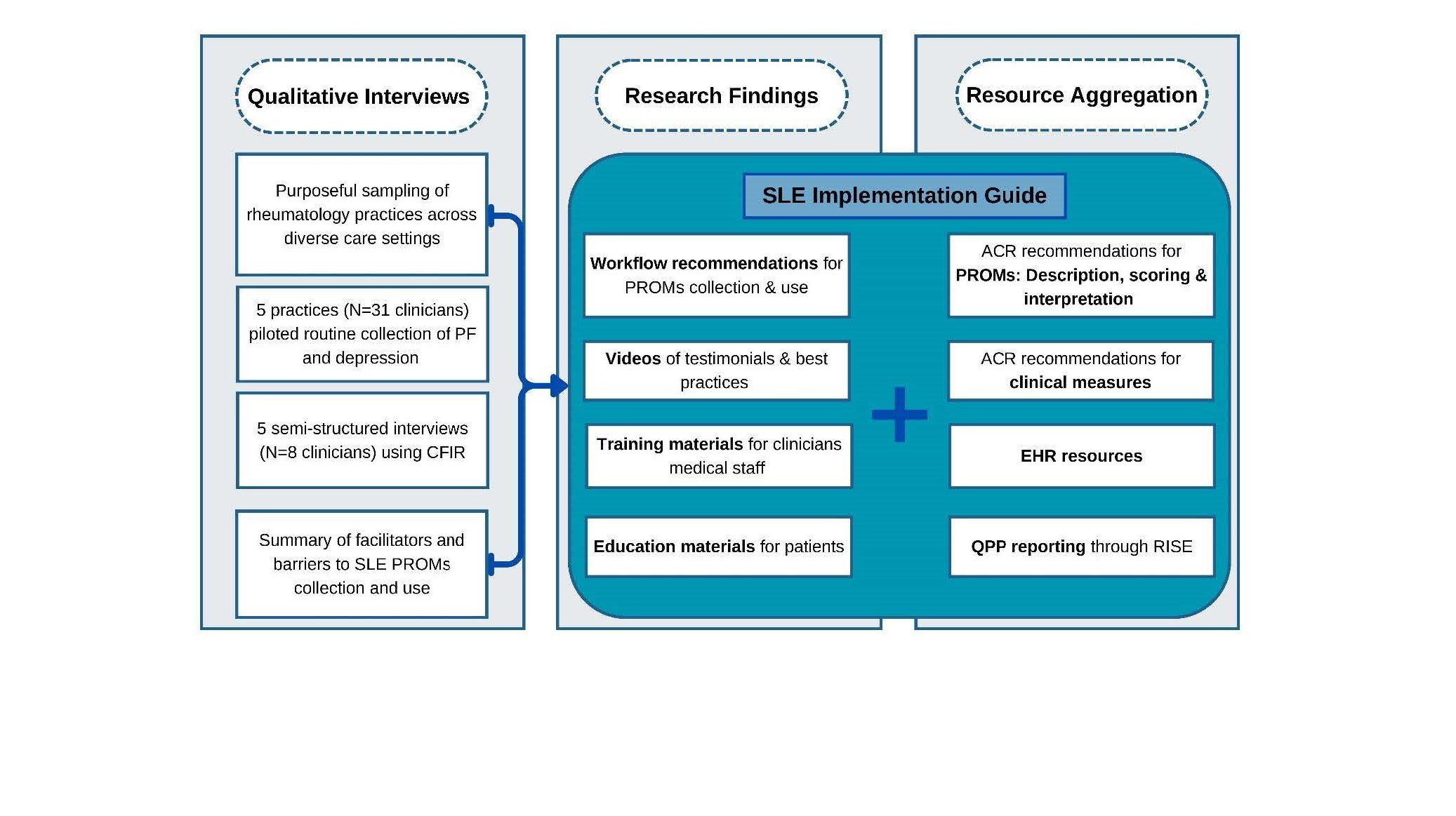 Figure1: Workflow for Developing the SLE Implementation Guide
Figure1: Workflow for Developing the SLE Implementation Guide
.jpg) Table 1. Characteristics of Pilot Practices.
Table 1. Characteristics of Pilot Practices.
.jpg) Table 2: Barriers and Opportunities of SLE PROMs Collection: Findings from Qualitative Interviews
Table 2: Barriers and Opportunities of SLE PROMs Collection: Findings from Qualitative Interviews
To cite this abstract in AMA style:
Nasrallah C, Hariz C, Kasturi S, Machua W, Yazdany J, Bartels C, Chiseri K, Blanks S, Katz P, Wilson C, Jorge A, Radtke B, Tack T, Garg S. Development of the American College of Rheumatology Implementation Guide for Integrating Patient Reported Systemic Lupus Erythematosus Quality Measures [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-of-the-american-college-of-rheumatology-implementation-guide-for-integrating-patient-reported-systemic-lupus-erythematosus-quality-measures/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-the-american-college-of-rheumatology-implementation-guide-for-integrating-patient-reported-systemic-lupus-erythematosus-quality-measures/
